
Glassy Carbon-Based Microelectrodes Array for Electrochemical Detection of Neurotransmitters
2George Mason University, USA
Background: Neurotransmitters detection can help diagnosing and monitoring various neurological disorders. For instance, Parkinson’s disease is characterized by dopamine decrease in the brain. As dopamine is electrochemically active, electrochemical lab-on-a-chip are excellent candidates for dopamine’s real-time detection. However, the cerebrospinal fluid contains interfering molecules that can interact with the electrode and diminish its electrochemical activity. Here we present the development of a microelectrodes array fabricated from glassy carbon—a biocompatible material that is highly resistant to molecules adsorption and has a large electrochemical window that can be integrated in electrochemical lab-on-a-chip.
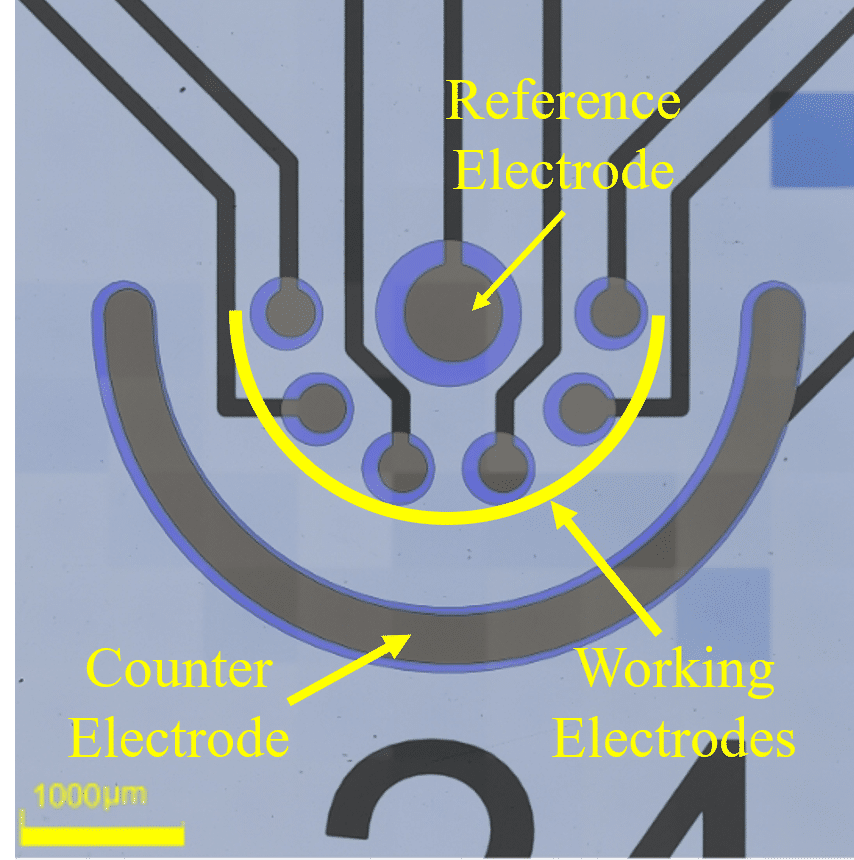
Methods: We used microfabrication techniques to pattern an electrochemical micro-chamber composed of microelectrodes made of SU8 3005 photoresist (Fig.1). The microelectrodes were pyrolyzed at 900°C to produce carbon conductive electrodes. The active surface area of microelectrodes was defined by an additional insulating layer of SU8. The pyrolysis optimization included the duration (1,2 and 4 hours) and the ramp rate (5,10 and 15C°/hour) of the process and their effect on the maximum electrochemical current. The electrochemical activity of the fabricated microelectrodes was characterized with the conventionally used ferricyanide/ferrocyanide redox couple.
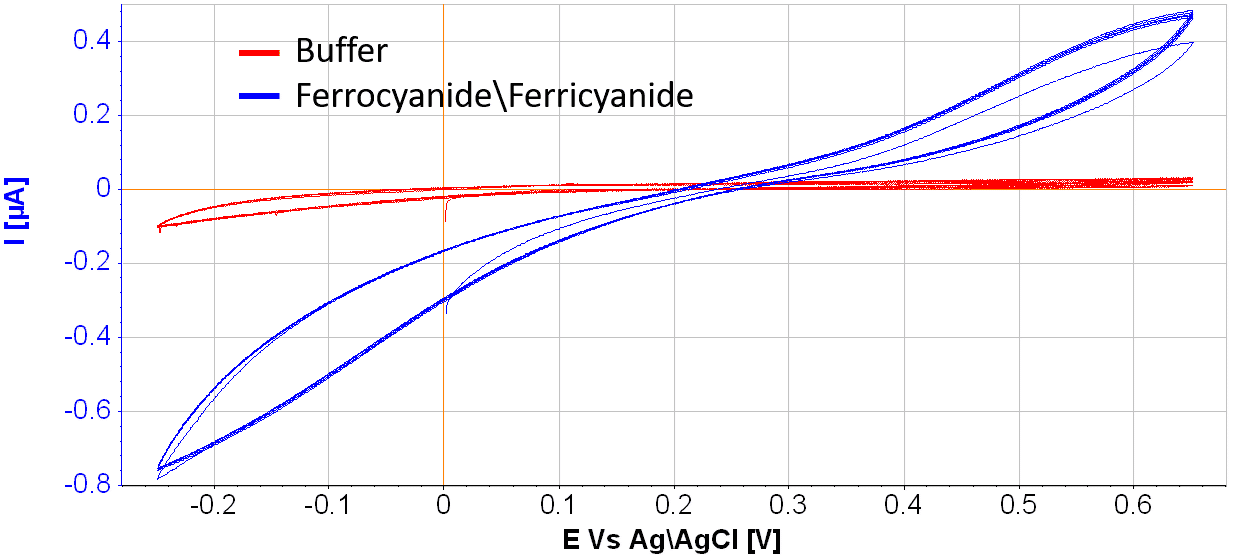
Results: Pyrolysis of 1-hour duration at a 10C°/hour rate in a nitrogen atmosphere resulted in the optical process parameters. The measured electrochemical signal (Fig.2) demonstrated increasing anodic (E>0.5V) and cathodic (E<0V) currents.
Conclusions: The fabricated glassy carbon microelectrodes were electrochemically active and can be used for analyte detection.
Powered by Eventact EMS