
Magnesium for UV Plasmonics and Chemical Reaction Sensing
We fabricate magnesium nanostructures with relatively strong resonances in the UV-region. Furthermore, we optically monitor chemical reactions taking place on plasmonic nanoparticles of Mg and other materials when exposed to various controlled gas compositions.
Plasmonic nanoparticles composed of common metals such as gold and silver exhibit resonances in the visible, near-infrared, and even terahertz spectral regions, enabling a multitude of applications ranging from chemical sensing to surface enhanced Raman and infrared spectroscopy. Such structures sustain localized surface plasmon resonances (LSPRs) which can be tuned throughout this region via various parameters, such as the shape and size of the particle. As the lateral size of a plasmonic antenna or its surrounding refractive index increases, so does its resonance wavelength. On the lower-wavelength side of the spectrum, however, pushing the limits of plasmonic applications is much more difficult, as the minimum wavelength at which a particle can sustain LSPRs is limited by the bulk plasma frequency of the utilized material. A material which has been shown to be capable of sustaining LSPRs at below 300 nm is aluminium; however, these resonances are relatively weak, and the chemical instability of Al is another problem which fuels the need for new materials that can be nanostructured to operate in the UV region.
As pointed out by Sanz and Ortiz [1], a promising candidate for this challenge is magnesium, which can theoretically sustain LSPRs below 350 nm, at intensities comparable to those found for silver. For this reason, we fabricated magnesium nanoantennas via colloidal hole-mask etching as well as electron beam lithography and subsequent electron beam physical vapour deposition (Figure 1a). The plasmonic behaviour of these structures is investigated via bright-field extinction spectroscopy on large areas of structures as well as via dark-field scattering spectroscopy on single particles.
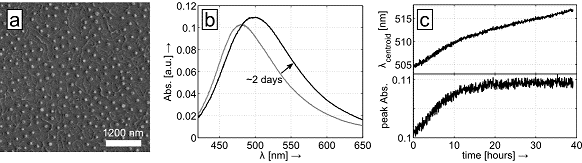
Figure 1: (a) SEM image of magnesium disks (40 nm in radius, 80 nm in thickness) fabricated via colloidal hole-mask etching.
(b) A resonance at around 480 nm is measured after evaporation, which increases in intensity and redshifts by tens of nanometers over the course of approximately two days of air exposure. (c) Characteristic reaction trajectory composed of centroid wavelength and maximum absorbance versus time.
As Mg is a highly reactive metal, Mg nanostructures are very well suited for all-optical investigation of the plasmonic response to chemical reactions taking place on the particle surface, such as oxidation, carbonization, and hydrogen uptake and release. We continuously record the spectrum of Mg particles during air exposure (Figure 1b), both in bright-field ensemble measurements as described by Schwind et al. [2], and extend this approach to single-particle dark-field scattering spectroscopy of individual Mg nanoantennas. Plotting the centroid wavelength and the maximum intensity of the resonance versus time yields a ‘reaction trajectory’ as shown in Figure 1c, which exhibits several intriguing properties. Here, we focus on a characteristic bend after a certain reaction time, which is potentially caused by the initial formation of a closed layer of reaction product on the NP surface, after which the reaction continues at a different rate. The increasing resonance intensity and wavelength observed in the measurement of Figure 1 is attributed to the refractive index of the forming oxide layer, which is significantly higher than that of air. This process competes with the decreasing size of the metal antenna and apparently outperforms it in the first stages of the reaction. Our work will lead to the characterization and analysis of chemical reactions occurring on NPs of different materials (Mg, Al, Ag, Cu, etc.) when exposed to atmospheres of different gas compositions at varying temperatures and pressures.
[1] J. Sanz and D. Ortiz, “UV plasmonic behavior of various metal nanoparticles in the near-and far-field regimes: Geometry and substrate effects,” J. Phys. Chem. C 117, 19606-19615 (2013).
[2] M. Schwind, S. Hosseinpour, C. Langhammer, I. Zoric, and C. Leygraf,“ Nanoplasmonic Sensing for Monitoring the Initial Stages of Atmospheric Corrosion of Cu Nanodisks and Thin Films”, J. Electrochem. Soc. 160, C487-C492 (2013).
f.sterl@pi4.uni-stuttgart.de
Powered by Eventact EMS