
Photoactivation of a Nanobiocomplex: a Novel Photodynamic Therapy of Cutaneous Melanoma
2Department of Human Molecular Genetics and Biochemistry, Tel Aviv University
Cutaneous melanoma (CM) is the most aggressive and deadliest form of skin cancer worldwide.
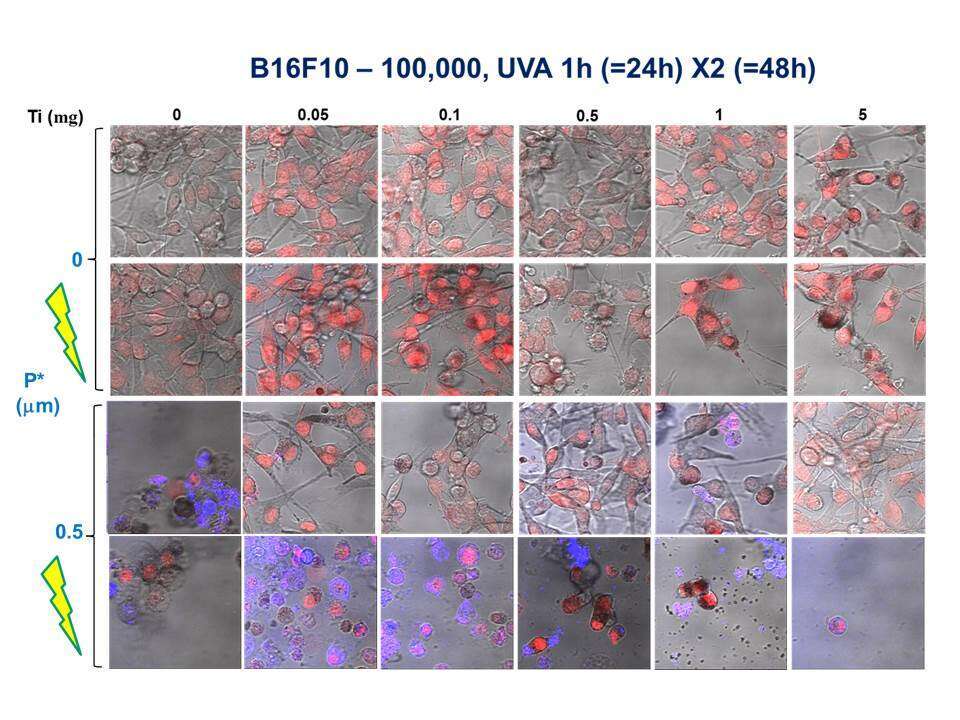
The cytotoxic effect of photo-excited titanium dioxide (TiO2) by UV illumination, creating reactive oxygen species (ROS), has been examined in several cancer models in vitro. However, serious damage to the surrounding healthy tissue limits the applicability of this approach. Our group has discovered a unique protein that strongly binds TiO2. This protein, dihydrolipoamide dehydrogenase (DLDH), is critical for energy and redox balance in the cell. It has been reported in the literature that ROS are generated as a result of its oxidative activity. CM cancer cells overexpress the cell surface receptor avb3 integrin, which interacts with proteins of the extra cellular matrix (ECM) through an RGD (Arg-Gly-Asp) recognition site. We bio-engineered the human DLDH with RGD tails on both sides (DLDH-RGD2), thus generating a protein capable of serving as a bridge between the integrin expressing cancer cells and TiO2 nanostructure forms. Activation of this nanobiocomplex by UVA illumination produces a synergistic toxic effect leading to enhanced cancer cell death (Fig 1).
We believe that the understanding gained from this work will also be beneficial to other integrin-expressing tumor models that have not been tested so far.
Powered by Eventact EMS