
Synthetic Environments to Understand Cancer Metastasis and Drug Resistance
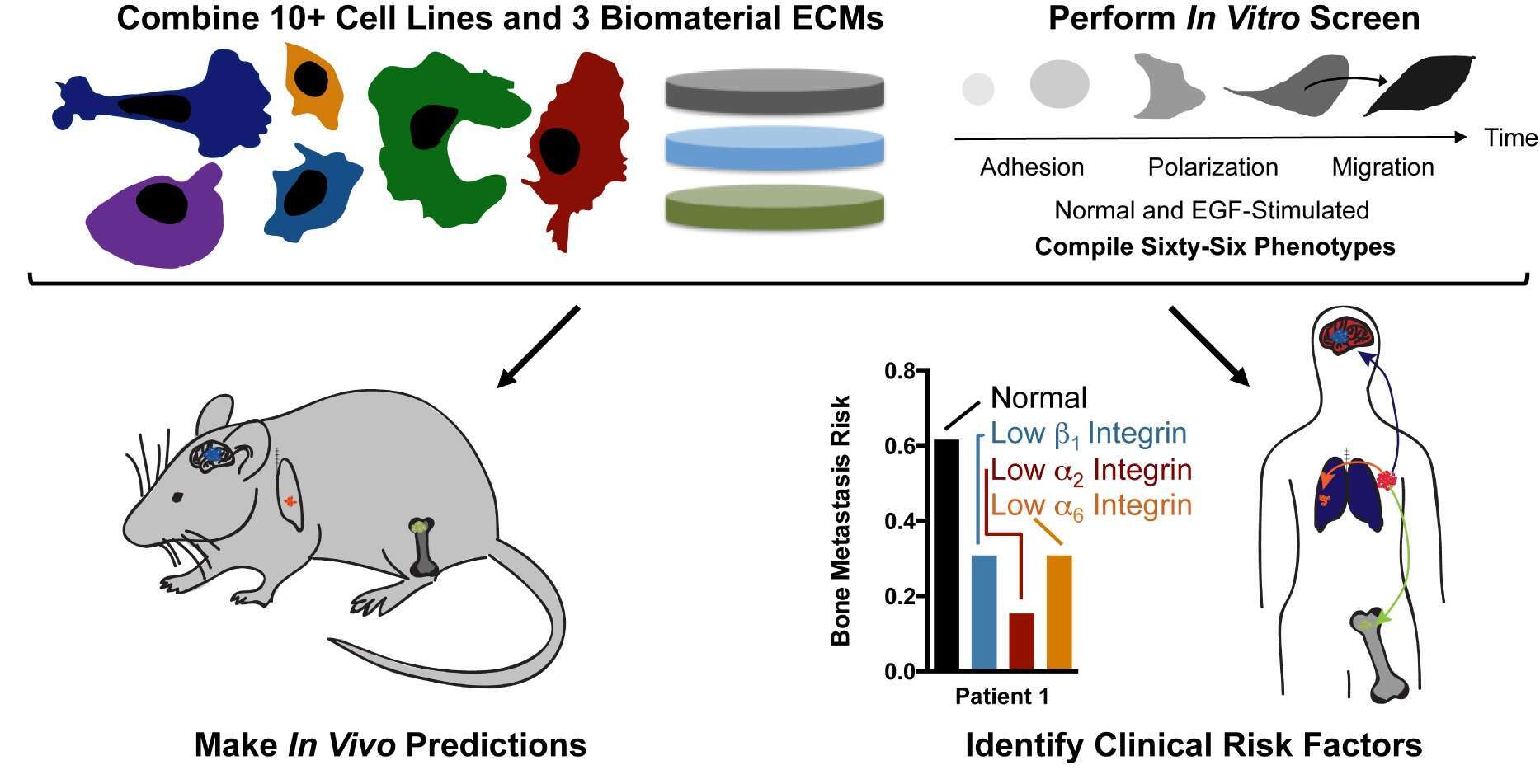
Metastasis is the leading cause of fatality for women diagnosed with breast cancer. The most common anatomical sites of distant tumor growth include the brain, lung, liver, and bone, and it is well known that this metastatic spread in breast cancer is not random. Rather, different clinical subtypes of breast cancer exhibit unique patterns of metastatic site preference, called tissue tropism. Given the physical and chemical diversity of these secondary tissue sites, my lab hypothesizes that there is a relationship between the biophysical and biochemical properties of the tissue, and the ability of cells within a particular subtype of breast cancer to adhere, migrate, grow, and respond to chemotherapeutics at these secondary sites. We created biomaterial microenvironments, which capture some of the key physical and biochemical elements of the secondary site tissues often recipient of breast cancer spread (brain, lung, and bone). Our approach is revealing how cell-material interactions are predictive of metastatic spread and non-canonical signaling pathways involved in drug resistance at these tissue sites. First, we can use a cell-ECM screening method in vitro to predict where a cell will metastasize in vivo (Barney et al. 2015). Second, we have demonstrated that a stiff tumor microenvironment reduces sorafenib treatment efficacy, which can be abrogated via JNK inhibition (Nguyen et al 2014). We will discuss these and current efforts toward biomaterial capture of dormant metastatic cells, rapid tumor spheroid formation, and the role of mesenchymal stem cells in drug resisatnce. We propose that these types of biomaterial environments can be used to predict tissue-specific metastasis, and may serve as a system that pharmaceutical companies can use to rule out false positives and potentially save billions of dollars in the drug development pipeline.
Powered by Eventact EMS