
BRINGING CELL THERAPY UP TO PATIENT BED SIDE – THE DEVELOPMENT OF AN AUTOMATED AND CONTROLLED POINT OF CARE CELL THAWING DEVICE
2Engineering, Pluristem Therapeutics Inc., Haifa
Unlike traditional drugs, cellular therapy process is not over until the thawed product is injected to the patient. It is well known that the thawing procedure involves stressful conditions to the cells. These conditions may cause or promote events such as cell injury or death. Normally vials thawing procedure is based on manual swirling the vial in a preheated (370C) water bath. This method is not controllable, reputable or robust and can lead to product inconsistency. Furthermore, the water bath thawing method requires the use of water in contact with the product package, which increases the risk of contamination and cannot be used in settings such as operating rooms.
In order to achieve control over the process up to the patient bed side, Pluristem used a QBD based approach in order to solve the problem and keep patient safety, product quality and ease of use in mind. We developed an easy to use and automated point of care thawing device (Fig 1) that mimics the standard thawing method (Fig 2) and controls thawing parameters such as temperature, agitation (Fig 3) and time. This device offers a repeatable, robust and simple thawing method that can be operated in clean rooms or clinics. Furthermore, the device futures the opportunity to log the data and insure cold chain integrity. The talk will describe the development strategies, pre and in process controls considerations as well as the validation of the device on Pluristem’s PLX (PLacental eXpanded) cell and other MSC’s cell types.
Figure 1: Point of care thawing device
Figure 2: Comparison of post thaw viability
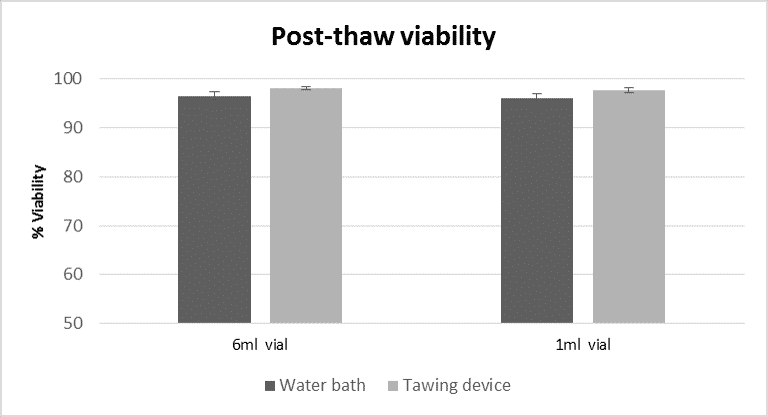
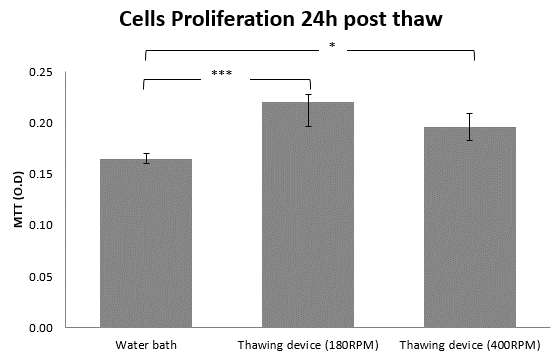
Figure 3: the effect of shaking speed on cell proliferation 24h post thaw
Powered by Eventact EMS