
Mutant p53 Modulates the Signal of Stromal-Secreted Hepatocyte Growth Factor (HGF) to Endow Cancer Cells with Drug Resistance
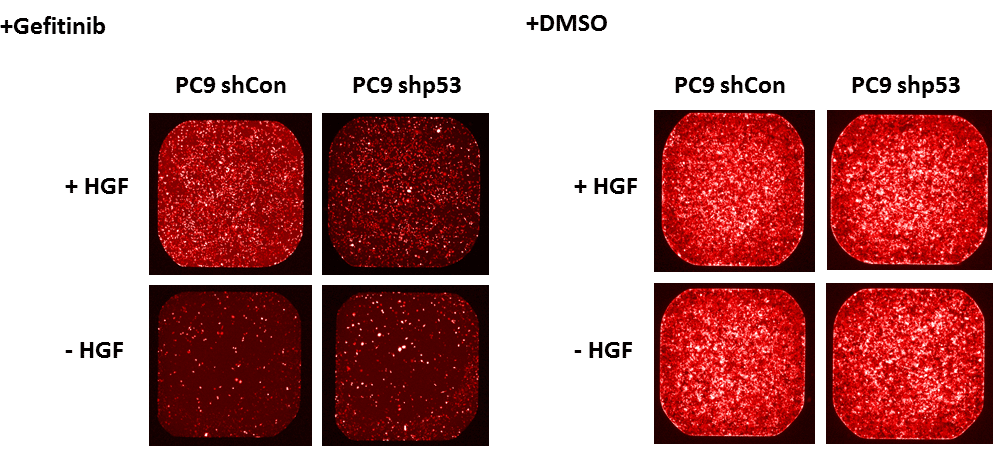
Drug resistance is a major obstacle in cancer therapy. Recently, a co-cultivation screen identified a major role of the microenvironment in promoting drug resistance in various solid tumors1. Mutations in the p53 tumor suppressor protein are highly frequent in tumors and often endow cells with tumorigenic capacities. However, the role of mutant p53 in the context of tumor-stroma interaction has not been thoroughly studied yet. In this study, we performed a cytokine screen, to identify potential secreted molecules which endow the cancer cells with drug resistance, in a mutant p53 dependent manner. We established isogenic lung cancer sub-lines, harboring different p53 statuses and labeled with tRFP. After treating these sub-lines with EGFR inhibitor and a cytokine library, we identified several potential cytokines which endow the mutant p53 expressing sub-line with enhanced resistance compared to mutant p53-knocked down sub-line. Remarkably, the most significant factor endowing drug resistance in a mutant p53 dependent manner was hepatocyte growth factor (HGF, see attached figure) , which was previously shown to be a major contributor to innate resistance to RAF inhibitors1. We therefore demonstrate a potential role for mutant p53 in modulating stromal-derived signals to promote drug resistance in the tumor cells. In a broad sense, we demonstrate how a cancer cell determinant can manipulate a stromal signal for the tumor’s benefit.
Reference
- Straussman, R. et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature 487, 500–4 (2012).
Powered by Eventact EMS