
SELF-ASSEMBLY OF AZIDE CONTAINING DIPEPTIDES
2The Center for Nanoscience and Nanotechnology, The Hebrew University of Jerusalem, Jerusalem
In nature, complex functional structures are formed by the self-assembly of biomolecules such as amino acids, nucleic acids and phospholipids under mild conditions. Understanding the forces that control self-assembly and mimicking this process in vitro will bring to major advances in the area of materials science.
Peptides, specifically, hold a great promise as biomolecular building blocks since they present substantial diversity, their synthesis in large scale is straightforward, and they can be easily modified with biological and chemical entities.
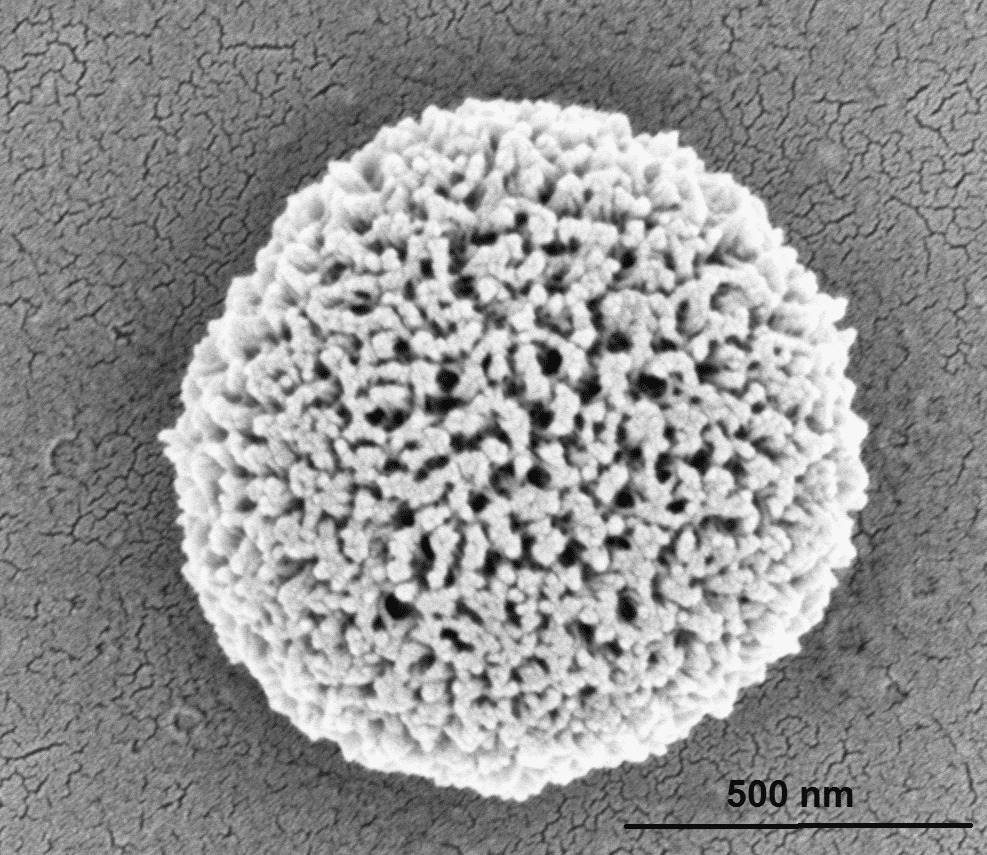
We present the self-assembly of three aromatic dipeptides containing an azide moiety: H-Phe(4-azido)-Phe(4-azido)-OH, H-Phe(4-azido)-Phe-OH, and H-Phe-Phe(4-azido)-OH. The peptide H-Phe(4-azido)-Phe(4-azido)-OH self-assembled into porous spherical structures, whereas the peptides H-Phe(4-azido)-Phe-OH and H-Phe-Phe(4-azido)-OH did not form any ordered structures under the examined experimental conditions. The azido group of the peptide can serve as a photo cross-linking agent upon irradiation with UV light. To examine the effect of this group and its activity on the self-assembled structures, we irradiated the assemblies in solution for different time periods. Using electron microscopy, we determined that the porous spherical assemblies formed by the peptide H-Phe(4-azido)-Phe(4-azido)-OH underwent a structural change upon irradiation. Moreover, using indentation experiments with atomic force microscopy, we showed that the Young`s modulus of the spherical assemblies increased after irradiation with UV light. These ordered assemblies or their peptide monomer building blocks can potentially be incorporated into other peptide assemblies to generate stiffer and more stable materials or to serve as a functional group and react easily to bind a variety of materials.
Powered by Eventact EMS