
DIRECTED EVOLUTION OF A SOLUBLE HUMAN IL-17A RECEPTOR FOR THE INHIBITION OF PSORIASIS PLAQUE IN MICE MODEL
2National Institute for Biotechnology in the Negev (NIBN), Ben-Gurion University of the Negev, Beer-Sheva
3Innovative Ventures Division, Teva Pharmaceutical Industries, Netanya
IL-17 is a T-cell-derived cytokine that promotes inflammatory pathology in autoimmune diseases. Blocking IL-17A interactions with its endogenous IL-17 receptor (IL-17RA) can constitute an important target for therapeutic intervention. Here, we utilized a directed evolution approach to generate soluble IL-17RA mutants exhibiting increased IL-17A binding affinity and thermostability, relative to the WT. Human fibroblast cell-based assay and in vivo analysis in mice indicated that two improved IL-17RA mutants efficiently inhibit the secretion of IL-17A-induced pro-inflammatory cytokines. Analysis of one of these mutants in psoriasis mice model showed its efficacy in promoting the recovery of psoriasis plaques. This mutant can be used as a promising drug candidate for the treatment of psoriasis and can be examined as a therapeutic agent for various other autoimmune diseases.
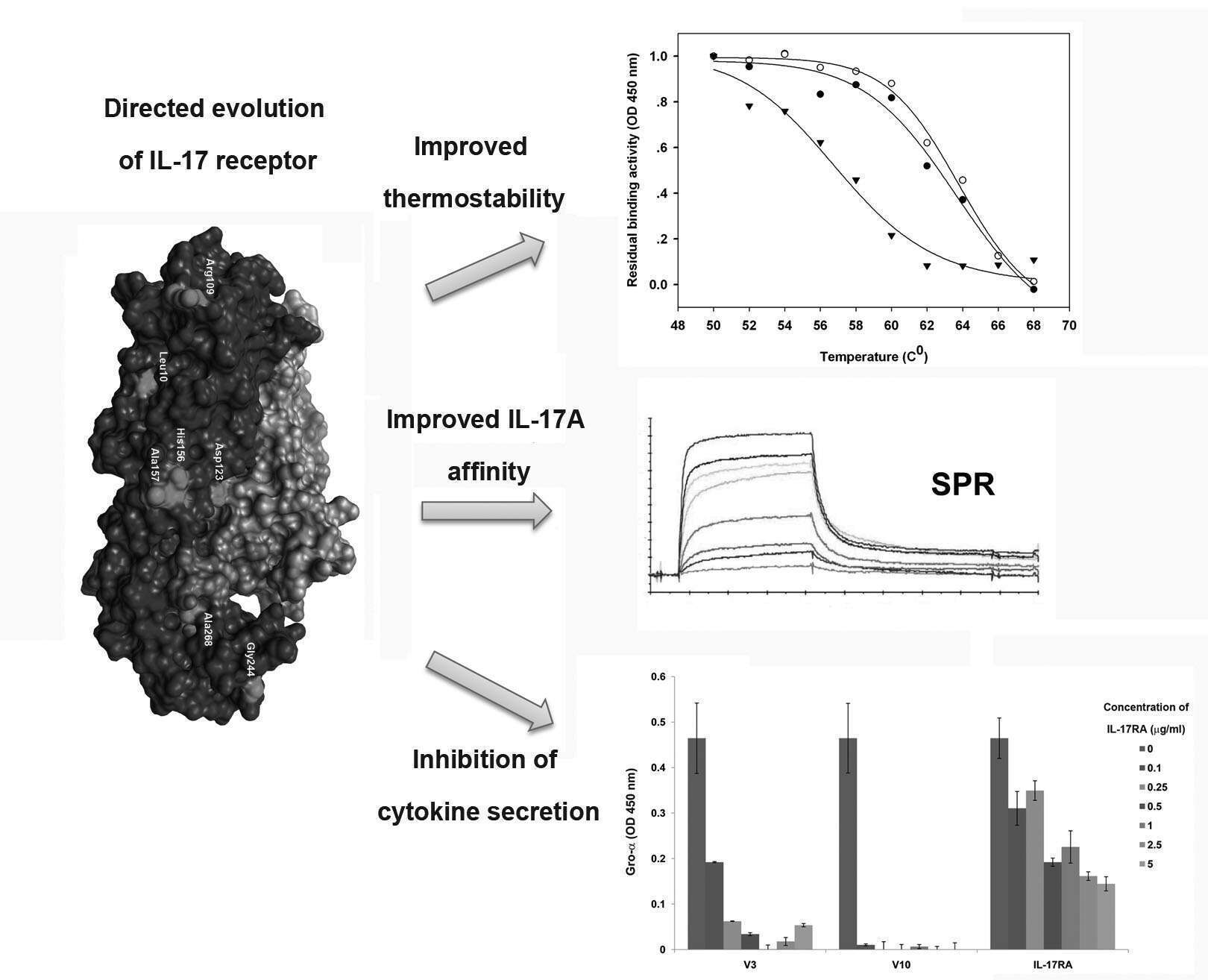
Zaretsky, M., Etzyoni, R., Kaye, J., Sklair-Tavron, L. & Aharoni, A. (2013). Directed evolution of a soluble human IL-17A receptor for the inhibition of Psoriasis Plaque Formation in a Mouse Model.
Powered by Eventact EMS