
THE 3D STRUCTURE OF GAN4C, A GH4 6-PHOSPHO-BETA-GLUCOSIDASE FROM GEOBACILLUS STEAROTHERMOPHILUS
2Department of Biotechnology and Food Engineering, Technion-Israel Institute of Technology, Haifa
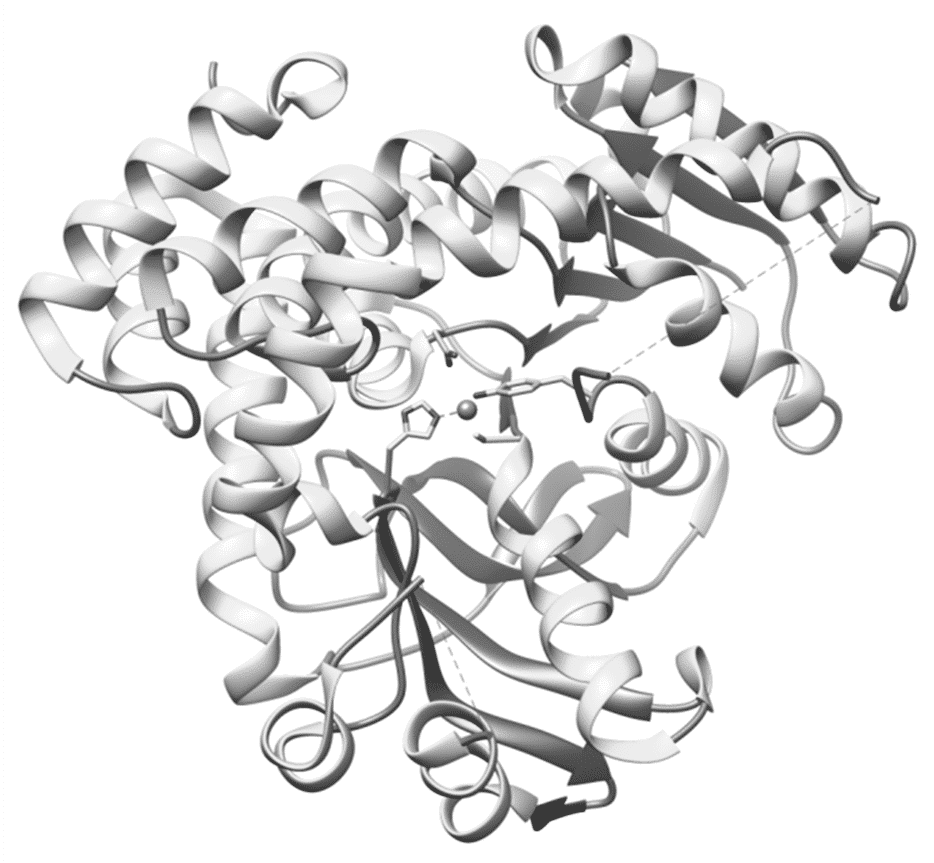
Geobacillus stearothermophilus T-6 is a thermophilic soil bacterium possessing an extensive and highly-regulated system for the utilization of xylan, arabinan and galactan. We have recently characterized the galactan-utilization gene cluster in this bacterium, containing a three-component regulatory system (GanPST), an ABC sugar transport system (GanE2F2G2), a transcriptional regulator (GanR2), a putative GH1 6-phospho-b-galactosidase (Gan1D), and a GH4 6-phospho-b-glucosidase (Gan4C). 6-Phospho-β-glucosidases are enzymes that hydrolyze the β-glycosidic bond between a terminal non-reducing glucose-6-phosphate and other organic molecules. These enzymes are currently classified into GH families 1 and 4 (GH1 & GH4), where the GH1 6-phospho-β-glucosidases act by a classical retaining mechanism, while the GH4 enzymes act by an unusual mechanism involving a divalent metal and NAD+ as a cofactor. To determine the structural basis for the unique mechanism of GH4 6-phospho-β-glucosidases, we investigated and report here the 3D structure of Gan4C. The structure of the Gan4C monomer adopts the characteristic dinucleotide-binding Rossman fold, containing specifically 12 b-strands that form two central b-sheets, which are surrounded by 17 a-helices. At the center of this protein monomer we found a manganese ion (Mn+2), coordinated to four different amino acid side chains, Tyr, His, Cys and Asn. In the crystal structure, the Gan4C monomer assembles as a dimer of dimers, forming a unique tetrameric structure, with an inner cavity of approximately 20Å in diameter. The current 3D structure and its comparison to related GH4 enzymes are used as a basis for more generalized conclusions concerning the structure-activity aspects in the GH4 family.
Powered by Eventact EMS