
BACK TO BASICS: CHALLENGING BOND ACTIVATION USING PALLADIUM COMPLEXES WITH LOW PERCENT BURIED VOLUME NHCS
The transition metal (TM) catalysed regio/stereoselective bis-silylation of alkynes has resulted in the formation of high value organosilicon compounds.[1] Platinum group metal complexes act as stoichiometric/catalytic mediators of this process but are largely limited to the use of strained or activated disilanes.[2] A vital mechanistic step is the oxidative addition of a disilane to the TM centre. Hexamethyldisilane represents one of the hardest Si-Si bonds to cleave and even stoichiometric examples using platinum group metals are rare.
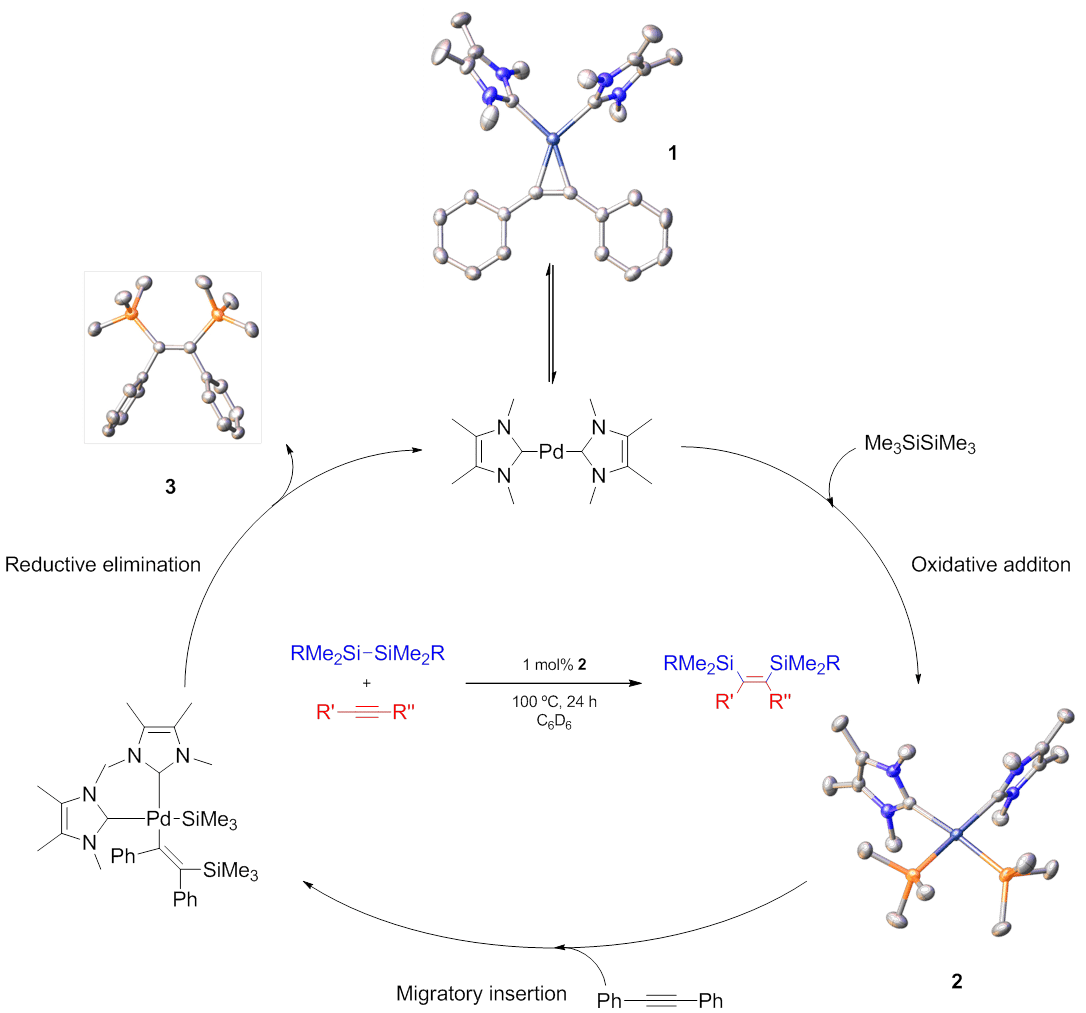
We have accomplished and fully characterised the first oxidative addition of hexamethydisilane to a palladium metal centre using (ITMe)2Pd(0) (ITMe = 1,3,4,5-tetramethylimidazol-2-ylidene).[3] The resulting complex, cis-Pd(ITMe)2(SiMe3)2, acts as a pre-catalyst for the bis-silylation of sterically and electronically demanding internal acetylenes using non-activated disilanes at low catalytic loadings. The reactivity of these complexes in this and other challenging bond activations will be presented.
[1]. a) M. Suginome, Y. Ito, J. Chem. Soc., Dalton Trans. 1998, 1925; b) M. Suginome, Y. Ito, Chem. Rev. 2000, 100, 3221; c) I. Beletskaya, C. Moberg, Chem. Rev. 1999, 99, 3435.
[2]. a) W. Finckh, B.-Z. Tang, A. Lough, I. Manners, Organometallics 1992, 11, 2904; b) F. Ozawa, M. Sugawara, T. Hayashi, Organometallics 1994, 13, 3237-3243; c) M. Suginome, H. Oike, Y. Ito, Organometallics 1994, 13, 4148.
[3]. M. B. Ansell, D. E. Roberts, F. G. N. Cloke, O. Navarro, J. Spencer, Angew. Chem. Int. Ed. 2015, 54, 5588.
Powered by Eventact EMS