
BROOK REARRANGEMENT AS A TRIGGER FOR RING-OPENING OF STRAINED CARBOCYCLES
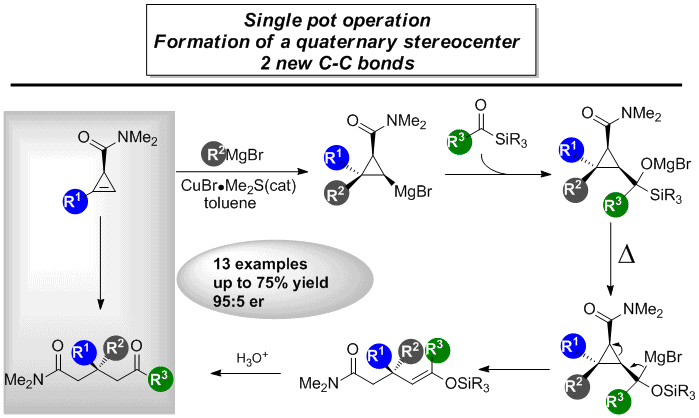
In organic synthesis, it has been a long-standing objective to construct valuable molecules from simple starting materials. In this context, over years our group has developed several efficient stereo- and enantioselctive strategies for C-C bond forming process in a single pot operation. Herein, we disclose a tandem diastereoselective carbometalation of cyclopropene, nucleophilic addition to acylsilane, Brook rearrangement and selective ring-opening of cyclopropane process in one pot operation. With this method, a variety of linear amides fragments bearing all-carbon quaternary stereogenic centres can be prepared in good yields form easily available cyclopropenyl derivatives. Enantiomerically enriched example and mechanistic proposal are also presented.
Powered by Eventact EMS