
RATIONAL DESIGN AND SYNTHESIS OF NOVEL PEPTIDOMIMETIC DRUG CANDIDATES FOR PROSTATE CANCER TREATMENT
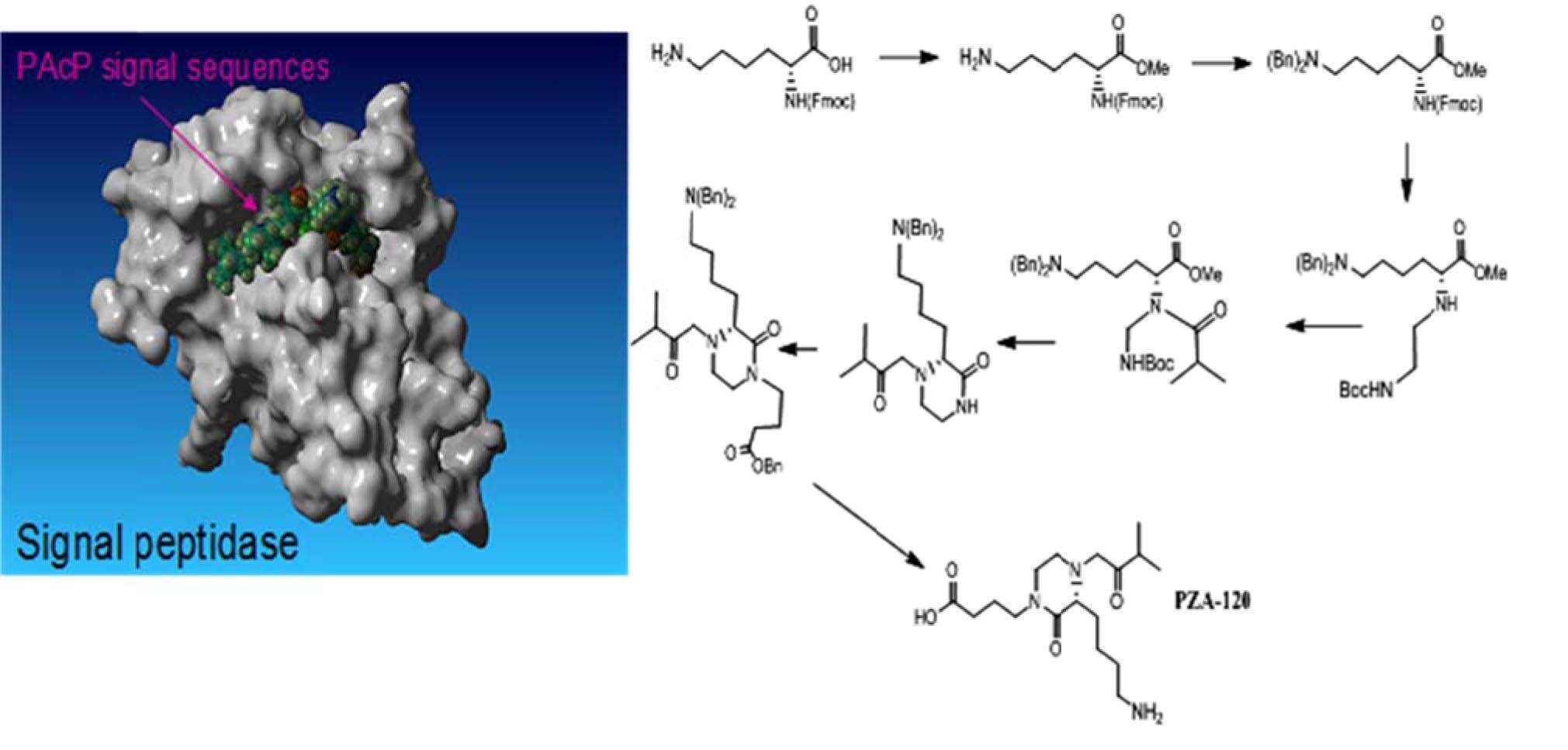
Despite progress in early diagnostics and treatment, prostate cancer still is one of the most devastating male diseases. Thus, the development of anti-prostate cancer drugs remains urgent. Prostatic acid phosphatase (PAcP) has been implicated in the pathogenesis of prostate cancer. In prostate epithelial cells PAcP is presented in two distinguished isoforms: extracellular and nuclear. These populations differ from each other by function, enzymatic activity and by protein folding. Secreted PAcP upregulates cancer growth of prostate epithelial cells (prooncogenic), whereas the nuclear form inhibits it (antioncogenic). We hypothesize that the primary factor that regulates the secretion of prooncogenic PAcP is appropriate its signal sequence cleavage. In fact, any secretory protein should lose its signal sequence in order to be properly secreted.
We established a new mechanism-based anticancer drug discovery approach for prostate cancer therapy: the inhibition of signal peptides 1 (SP1), the enzyme that cleaves PAcP signal sequence. Such inhibition should prevent the secretion of prooncogenic PAcP and slows prostate cancer progression. Using homology computer modeling, we built a virtual human SP1 model. Based on signal sequences of PAcP, a series of potential SP1 inhibitors, both peptide-based and peptidomimetics (novel (S)-3-(4-aminobutyl)piperazin-2-one derivatives) were designed and synthetized. One of synthetized molecules was active in vitro in prostate cancer model. The compound decreased the secretion of PAcP from prostate cancer cells and showed cytotoxic activity. Non-cancer cells were not affected by the compound. Reported in this study, innovative drug development approach which based on inhibiting PAcP secretion and in silico SP1 model was not previously described in the scientific literature.
Powered by Eventact EMS