
THE ROLE OF PEPTIDE SIDE CHAINS AND ORDER IN DETERMINING PROTON CONDUCTION OF SELF-ASSEMBLED CYCLIC PEPTIDE NANOTUBES
2The Ilse Katz Institute for Nanoscale Science & Technology, Ben-Gurion University of the Negev
The quest for the development of environmental friendly materials has accelerated research into the structure, chemistry and functionality of biomaterials in recent years. The use of peptides for such tasks is particularly attractive due to their chemical diversity, ease of synthesis and their unique propensity to self-assemble to form nanofibrils and nanotubes. Incorporating free amine side chains into the peptide sequences make those nanostructures proton accepting and hence can be used to promote proton conduction. Furthermore, the role of aromatic amino acids in proton transport in nature, suggests that these amino acids can be further used to induce proton conduction. The effect of aromatic amino acids on proton conduction in alternating D,L α-cyclic peptide nanotubes will be presented here.
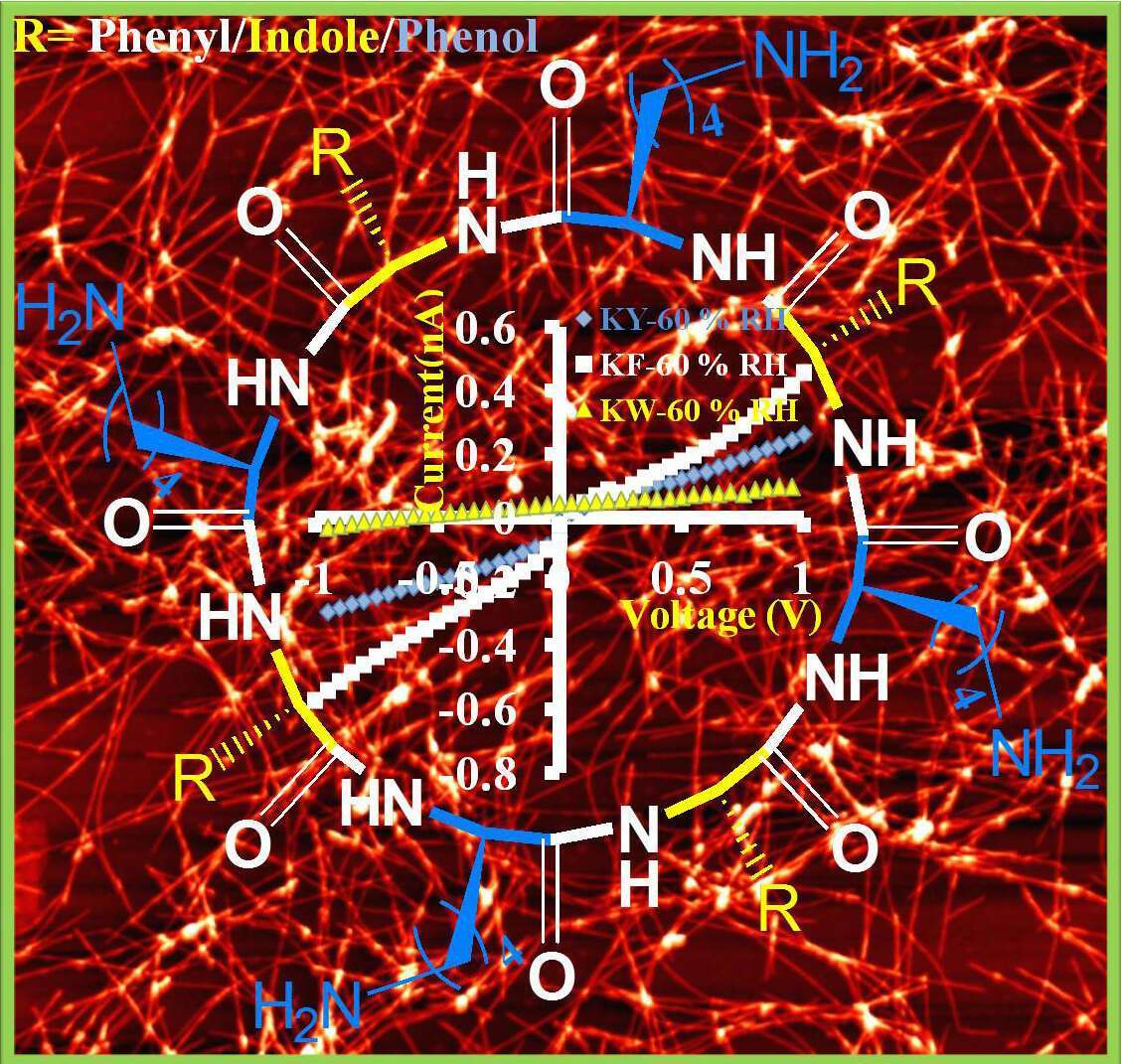
The designed octa alternating D,L cyclic peptides contain lysine amino acids in their four L-positions and one of the natural aromatic amino acids in their four D-positions, constructing three different cyclic peptides with a common formula c(KX)4 (X=Phe/Tyr/Trp). These alternating D,L α-cyclic peptides undergo self-assembly to form nanotubes under 1:9 water- acetonitrile mixture. Current-voltage measurements show an exponential dependence of the conductance on the relative humidity, as expected for proton conduction for each of the nanotubes. Surprisingly, c(KF)4 shows higher protonic conduction than that of the other two peptides [c(KY)4 and c(KW)4] under a high relative humidity range of 20-80 %. The observed higher protonic conduction is related to the very long nanofibriller network morphology of c(KF)4, indicating the importance of order in promoting proton transport. Overall, we show the possibility to tune proton conductivity by peptide design. Our findings may hold implications for the next generation of biocompatible proton-conducting materials for future protonic devices.
Powered by Eventact EMS