
CHARACTERIZATION OF POROUS MATERIALS FOR GAS SEPARATION USING DYNAMIC SORPTION TECHNIQUES
2Science, Quantachrome GmbH & Co. KG, Odelzhausen
Porous materials of various chemistries and especially pore network structure are becoming increasingly important for separation of gases for purification (of biogas for example) and sequestration. The underlying mechanism depends on the relative adsorption strengths of each species through available chemical bond formation, physical adsorption which is strongly dependent on pore size in terms of both enhanced adsorption due to fluid confinement effects, and molecular sieving effects.
Regarding the characterization of candidate materials, the practical importance of state-of-the-art dynamic (breakthrough) analysis is that it closes a fundamental gap in knowledge between static, equilibrium surface area (B.E.T.) and pore size measurements of porous materials and the determination of their effective sorption capacities under the dynamic conditions that are often found in industrial practice.
The adsorption of two environmentally important gases, CO2 and CH4, on a material suitable for industrial scale, has been investigated using equilibrium static isotherms, dynamic single component (in helium carrier gas) and admixture sorption studies as a function of pressure, flow rate and percent composition using a new fully automated apparatus. Kinetically controlled and thermodynamically controlled processes have been experimentally identified and modeled theoretically. The mass transfer zone and kinetic component which control the efficiency of the separation process, independent of equilibrium sorption capacity, are presented.
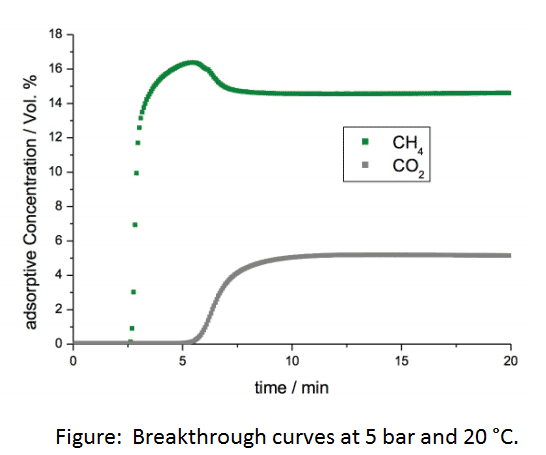
It is anticipated that these types of measurements will be of critical importance in the further development of hierarchical pore structure materials.
Powered by Eventact EMS