
DESIGN AND SYNTHESIS OF TRICYCLIC SPIROLACTONE TRPV1 AGONISTS
TRPV-1 is the Transient Receptor Potential cation channel, subfamily V, member 1. TRPV-1 receptor is associated with different diseases, such as migraine, cystitis, bone cancer, and many others. An efficient way to attenuate the pain caused by TRPV-1 is through desensitization of the receptor by continuous exposure to its agonists. The most potent TRPV-1 agonists are capsaicin, the active component of chili pepper, and RTX, toxin produced by Gram-negative bacteria.
Although capsaicin is a potent and readily available agonist it has the disadvantage of being a pungent compound. On the other hand, RTX is not accessible in large quantities due to the limited natural production and to its complicated total synthesis. Thus, the development of new, easily accessible agonists/antagonists of TRPV-1 is required.
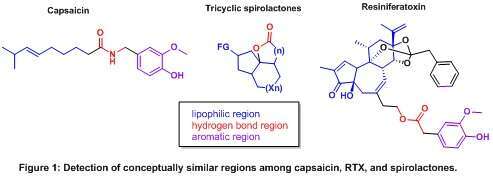
Numerous tricyclic angularly fused lactones, designed in our group, show remarkable structural similarities with capsaicin and RTX. As shown on Figure 1, the lactone moiety and the remaining bicyclic system match with the proton acceptor region and with the lipophilic area, respectively, whereas the aromatic part is absent. Thus, the tricyclic spiranoid lactones can be regarded as simplified RTX and capsaicin analogues, and therefore as potential TRPV1-active compounds.
The pool of 24 new-synthesized tricyclic spirolactones was subjected to in vitro biological studies (calcium imaging), and 11 showed agonists activity, as expected. 4 representative molecules were used as a platform for further manipulations towards the development of new TRPV1 agonists. The different aromatic moieties were integrated as a part of the new molecules, in order to increase their agonist activity. That led to the generation of a set of functionalized spiranoid lactones, which were subjected to in vitro and in vivo assays.
Powered by Eventact EMS