
Di-N METHYLATION OF ANTI-GRAM POSITIVE AMINOGLYCOSIDE-DERIVED MEMBRANE DISRUPTORS IMPROVES ANTIMICROBIAL POTENCY AND BROADENS SPECTRUM TO GRAM NEGATIVE BACTERIA
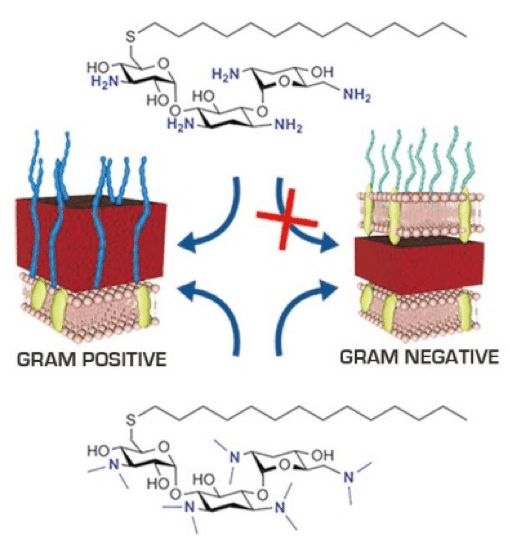
Antimicrobial cationic amphiphiles derived from aminoglycoside pseudo-oligosaccharide antibiotics interfere with the structure and function of bacterial membranes and offer a promising direction for the development of novel antibiotics. In this study, we report the effect of di-N-methylation of bacterial membrane disruptors derived from aminoglycosides (AGs) on antimicrobial activity. Di-N-methylation of cationic amphiphiles derived from several diversely structured AGs resulted in a significant increase in hydrophobicity compared to the parent compounds that improved their van der Waals interactions with membrane lipids. The antimicrobial properties of all di-N-methylated cationic amphiphiles were superior to those of clinically used bacterial membrane disrupting antibiotics in potency. The modification also broadened the spectrum of activity; whereas the parent compounds were active against Gram positive pathogens, di-N-methylated compounds were also active against Gram negative bacteria. The reported modification offers a robust strategy for the development of broad-spectrum membrane-disrupting antibiotics with potency against bacterial pathogens that have high levels of antibiotics resistance.
Powered by Eventact EMS