
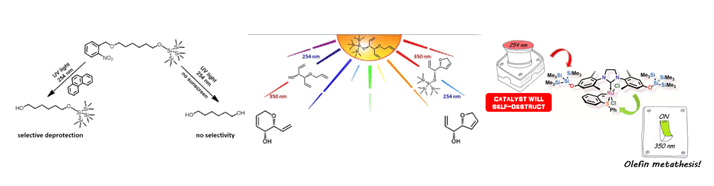
GUIDING CHEMISTRY WITH LIGHT
We chemists, are used to changing pH, temperature, concentrations and other factors in order to make chemical reactions happen, occasionally even in a selective fashion. The use of light in this regard, chromatic selectivity or orthogonality,1 is much less developed. Light is the most valuable and perennial of our energy resources. It has been regularly exploited by chemists and nature to promote specific chemical reactions; most rewardingly in the area of photocatalysis. In this talk I will show some recent progress made in our group in the area of selective light activated olefin metathesis catalysis, the ‘sunscreen’ effect in photochemistry and how we take advantage of chromatic orthogonality to guide chemistry by light.2
1. a) G. Bochet, Synlett. 2004, 13, 2268-2274; b) A. Blanc, C. G. Bochet, Org. Lett. 2007, 9, 2649-2651; c) C.-H. Wong, S. C. Zimmerman, Chem. Commun. 2013, 49, 1679-1695.
2. a) E. Levin, S. Mavila, O. Eivgi, E. Tzur, N. G. Lemcoff, Chem. Int. Ed. 2015, 54, 12384-12388; b) O. Eivgi, E. Levin, N. G. Lemcoff, Org. Lett. 2015, 17, 740-743; c) Sutar, R. L.; Levin, E.; Butilkov, D.; Goldberg, I.; Reany, O.; Lemcoff, N. G., Angew. Chem. Int. Ed., 2015,DOI: 10.1002/anie.201508966R1.
Powered by Eventact EMS