
STEREO- AND REGIOSELECTIVE SYNTHESIS OF TRICYCLIC SPIROLACTONES VIA DIASTEREOMERIC DIFFERENTIATION OF COLLECTIVE KEY PRECURSOR
Many important biochemical compounds and drugs of natural origin contain spirofuranone ring structures.1 Intriguingly, only several tricyclic combinations (most frequently composed of 5- or 6-membered ring sets) are observed; notably and to the best of our knowledge, no evidence of a natural 5/5/5 topology exists.
We were therefore motivated to develop a general and practical synthetic methodology for accommodating the construction of 5/5/5 core structures, with the view that such a platform, which is miniature, rigid, and closely resembles common natural scaffolds, may carry the potential for becoming a valuable fundamental component for the design of drugs and therapeutic agents.
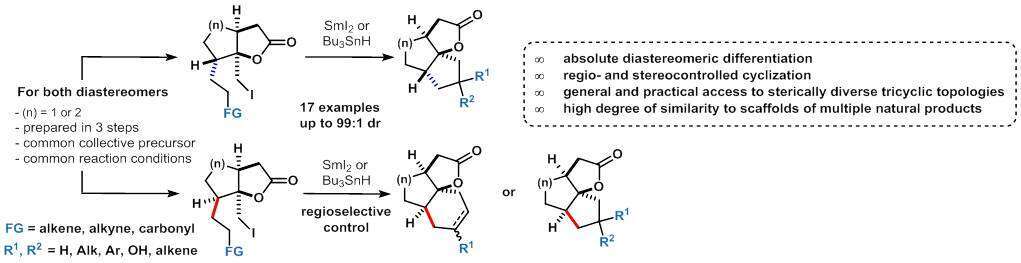
To design a basic platform, we employed an already established methodology previously designed by our group with the purpose of delivering less rigid, frequently occurring tricyclic spiranoid lactones such as 5/6/5, 6/6/5, etc.2 However, in the event, an interesting twist emerged and the final cyclization was found to be strictly dependent on the geometrical properties of the common precursor.
Thus, assemblies of diastereomeric iodolactones, designed from key cycloalkylmethylene precursors, are converted into a wide range of tricyclic angularly fused spiranoid lactones with different topologies by simple diastereomeric differentiation in a regioselective and stereodirected fashion.3
[1] (a) Dewick, P. M. Medicinal Natural Products: A Biosynthetic Approach. 2 ed.; Wiley, 2002. (b) Osbourn, A. E.; Lanzotti, V. Plant-Derived Natural Products. Springer, New York, 2009.
[2] Mostinski, Y.; Valerio, V.; Tsvelikhovsky, D. J. Org. Chem. 2015, 80, 10464.
[3] V. Valerio, Y. Mostinski, R. Kotikalapudi, D. Tsvelikhovsky, Chem. Eur. J. 2015, Manuscript Accepted.
Powered by Eventact EMS