
THE EFFECT OF ORGANIC LIGAND ON SILICON NANOPARTICLES AS ANODE MATERIAL FOR Li-ION BATTERIES
Batteries are valuable electrochemical cells and play an important role in the green energy strategies. Typical electrochemical cell is composed of many components that affect each other. In this study, we focus on the understanding the contribution of organic ligands decorating Si nanoparticles (NPs) surface. These organic ligands can affect the solid-electrolyte interface (SEI) formation, the insertion of the Li ion into the Si, and to improve the conductivity of the anode.
In order to decorate Si NPs surface with organic ligands, octyne, perfluorooctyne and triethoxy(octyl)silan molecules are used. The resulting materials are fully characterized by electrochemical measurements. In general, we have found that the octyne treatment does not affect the electrochemical properties. Moreover, since the fluoro-organic layer on the Si NPs changes the connection to the SEI, the Perfluorooctyne treatment has decreased the capacity and cycle ability of the Si anode due to the formation of wide cracks on the anode.
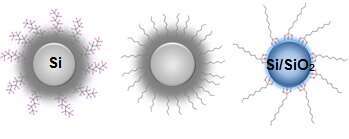
From left to right, Si NPs after perflorooctyne, octyne, and triethoxyoctyne silan treatment
Powered by Eventact EMS