
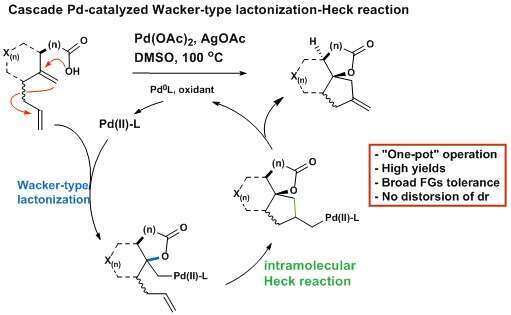
SYNTHESIS OF BI- AND TRICYCLIC SPIRANOID LACTONES VIA WACKER-HECK CASCADE CYCLIZATION
Palladium-catalyzed cascade reactions are used extensively in synthesis of highly functionalized carbo- and heterocycles.1 Very often, these transformations involve a reactive alkyl-Pd intermediate, which can undergo further transformations prior to a terminal reductive process. Depending on the reactants and the reaction conditions, it is possible to trap these transient alkyl-Pd species with alkenes, via the Heck-type C-C bond formation reaction. We realized that most of the reported cascade approaches rely on the Wacker cyclization of alcohols2, whereas, to the best of our knowledge, no cascade sequences involving a Wacker lactonization, followed by Heck type C-C bond formation has never been reported. In this perspective, an unprecedented intramolecular cascade cyclization of diene carboxylic acids leading to bi- and tricyclic scaffolds has been developed. This transformation involves oxidative Wacker type lactonization generating σ-Pd intermediate which is intercepted by alkene functionality via Heck reaction in a single transformation. This method provides rapid access to highly functionalized spiranoid lactones of different topology and stereochemical configurations. The transformation shows good functional group tolerability and broad substrate scope In addition, a rarely reported palladium-catalyzed 4-exo-trig Heck-type cyclizations are demonstrated by this protocol.
References: 1. (a) Nicolaou, K. C.; Edmonds, D. J.; Bulger, P. G. Angew. Chem. Int. Ed, 2006, 45, 7134; (b) Vlaar, T.; Ruijter, E.; Orru, R. V. A. Adv. Synth. Catal, 2011, 353, 809.; 2. Silva, Z. F.; Reiter, M.; Mills-Webb, R.; Sawicki, M.; Klär, D.; Bensel, N.; Wagner, A.; Gouverneur, V. J. Org. Chem. 2006, 71, 8390.
Powered by Eventact EMS