
PREPARATION AND CHARACTERIZATION OF ELUSIVE IODO(iii)ENOLATES AND THEIR USE IN C-CBOND FORMING REACTIONS
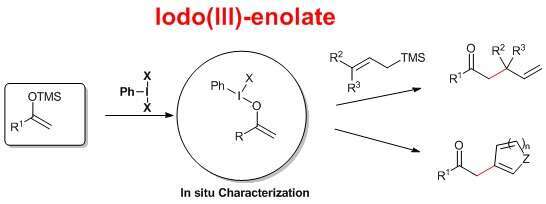
Iodo(III)-enolates have long been implied as intermediates in the hypervalent iodine mediated umpolung and alfa-functionalization of carbonyl compounds by nucleophiles.
Due to their instability and high reactivity these enolates have so far eluded characterization. Their preparation and use in synthesis has thus so far been limited to reactions in which the nucleophile could be included in the reaction mixture from the outset. Specifically, C-C bond forming reactions have been limited to simple enolate dimerization.
We now report a method for the preparation of iodo(III)enolates as discrete species. This for the first time allowed their in situ characterization and their use in C-C bond forming reaction such as direct allylation and arylation.
Powered by Eventact EMS