
FORMATION OF STEREODEFINED TRISUBSTITUTED SILYL ENOL ETHERS AS A NEW ROUTE TO CARBON QUATERNARY STEREOCENTERS
Formation of enantiomerically pure quaternary carbon center a to a carbonyl center remains a challenge in modern synthetic organic chemistry. The aldol reaction could provide an access to such molecular framework if stereodefined trisubstituted enolate could be formed stereoselectively1. To solve this problem, we have developed a one-pot method for the generation of stereodefined trisubstituted enolates from simple alkynes by a carbometalation/ oxidation sequence2 that was subsequently trapped as trisubstituted silyl enol ether 3. Using the Mukaiyama aldol reaction of these silyl enol ethers with aliphatic and aromatic aldehydes led to the formation of the aldol products possessing the expected quaternary stereocenter in high yield and diastereoselectivity [Scheme 1]3.
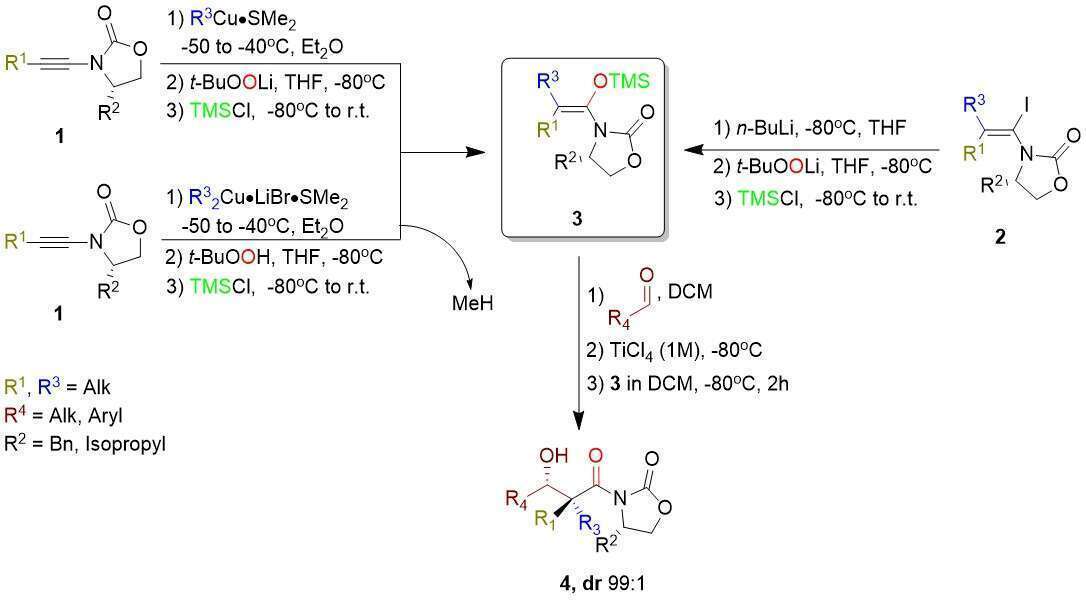
Scheme 1: The Mukaiyama addition with stereodefined trisubstituted silyl enol ether derivatives
[1] Y. Minko, I. Marek, Chem. Commun. 2014, 50, 12597-12611
[2] Y. Minko, M. Pasco, L. Lercher, M. Botoshansky, I. Marek, Nature, 2012, 490, 522-526.
[3] Z. Nairoukh, I. Marek, Angew. Chem. Int. Ed. DOI: 10.1002/anie.201507209.
Powered by Eventact EMS