
ALKYLATION OF STEREODEFINED TRISUBSTITUTED COPPER ENOLATE TOWARDS THE FORMATION OF CARBON QUATERNARY STEREOCENTRES
The formation of enantiomerically pure quaternary carbon stereocentres in acyclic systems represents one of the most significant challenges in modern synthetic organic chemistry. Preparation of stereodefined trisubstituted enolate could provide a solution, if they can be formed stereoselectively.1 To achieve this goal, we have developed a one-pot method for the generation of stereodefined trisubstituted enolates 2 from simple alkynes by a carbometalation/ oxidation sequence2. Addition of suitable electrophile led to the formation of the expected quaternary stereocentres in high yield and diastereoselectivity [Scheme 1]3.
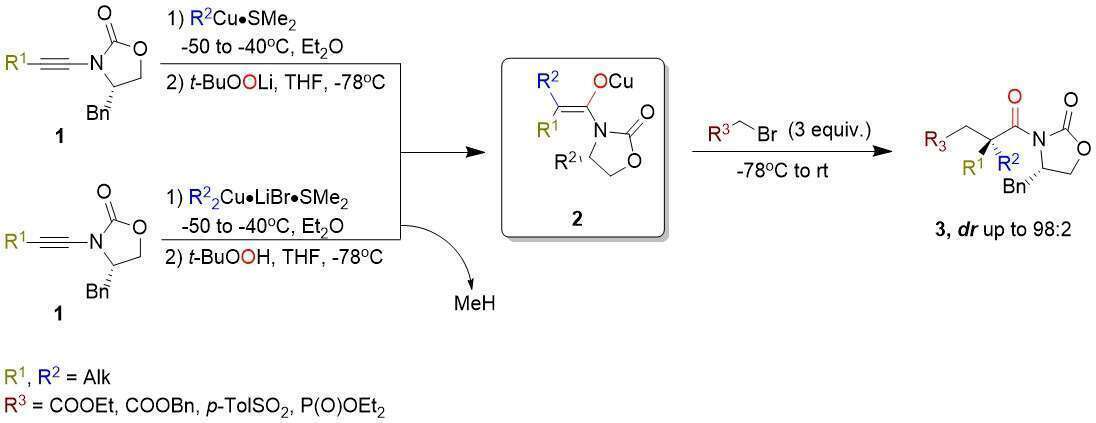
Scheme 1: Alkylation of stereodefined trisubstituted copper enolate
[1] Y. Minko, I. Marek, Chem. Commun. 2014, 50, 12597-12611
[2] Y. Minko, M. Pasco, L. Lercher, M. Botoshansky, I. Marek, Nature, 2012, 490, 522-526.
[3] Z. Nairoukh, Yury Minko, G. N. Kumar, I. Marek, Manuscript in preparation.
Powered by Eventact EMS