
SUPRAMOLECULAR ASSEMBLIES OF HOMOCHIRAL ALLENO-ACETYLENES: CATENANES AND HELICAL CAGES.
2Laboratory of Organic Chemistry, ETH Zurich
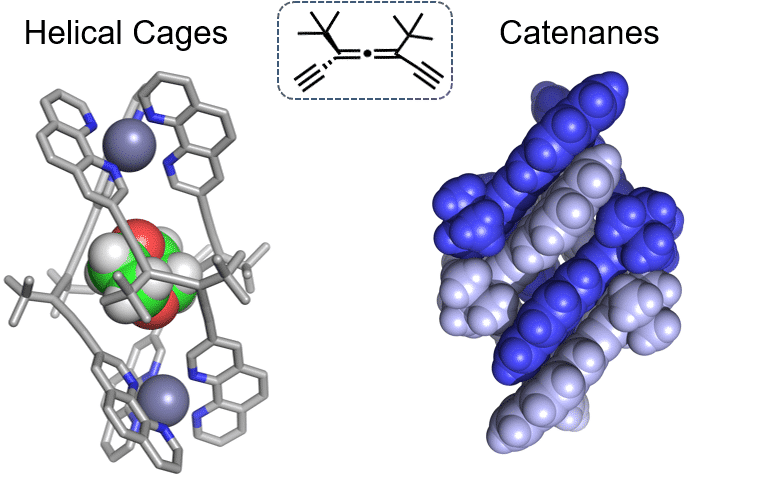
Helicates are a particularly interesting class of supramolecular assemblies, widely investigated for their elegant and intrinsically chiral structure. While the enantioselective interaction of helicates with large biological molecules is well known, examples of their function as receptors for small organic guests are rare, mainly due to the lack of a sufficiently large cavity. Here we describe the diastereoselective assembly of enantiopure alleno-acetylenic ligands to form triple-stranded helicates, which provide a sufficiently large helical cage (“helicage”) for the encapsulation of guests. The ECD spectra of the helicates, which showed strong Cotton effects and exciton coupling, were found to be extremely sensitive to the nature of the guest molecules. Consequently, a series of nonchromophoric, achiral guests of different sizes as well as regioisomers became distinguishable on the basis of their induced CD (ICD) spectra. Particularly high affinity towards 1,4-dioxane allowed its selective detection at parts-per-million (ppm) levels in aqueous solutions.[1]
The alleno-acetylenic helicates reversibly assemble to form mechanically interlocked molecules – catenanes. Particularly interesting is the assembly of enantiopure bis[2]catenane, containing 14 chirality centers. Highly selective narcissistic self-sorting was demonstrated for a racemic mixture consisting of both short and long alleno-acetylenic strands, highlighting their potential for the preparation of longer catenanes.[2]
[1] Gidron, O.; Jirasek, M.; Trapp, N.; Ebert, M-O.; Zhang, X.; Diederich, F. J. Am. Chem. Soc. 2015, 137, 12502–12505.
[2] Gidron, O.; Trapp, N.; Ebert, M-O.; Diederich, F. Angew. Chem. Int. Ed. 2014, 53, 13614-13618.
Powered by Eventact EMS