
QUANTITATIVE NMR DIFFUSION MEASUREMENT
NMR is an excellent technique for measuring diffusivity and for separating different compounds in a mixture. However, the nature of the experiment means that the intensity of the diffusion signals are strongly affected by relaxation effects.
NMR diffusion (Diffusion Ordered Spectroscopy – DOSY) is represented as a plot of diffusion rate against chemical shift. The projection on the diffusion axis resembles a chromatogram but the peak areas are not proportional to the concentration of the compounds.
To make the chromatogram-like projection quantitative, a diffusion and two relaxation (T1 and T2) spectra are combined. Each signal is evaluated for its chemical shift, line-width, diffusion rate and relaxation times. The signal height is then adjusted to compensate for the evolution of relaxation decay during the pulse sequence in a manner that improves on previous methods.1 The result is that the areas of the peaks in the projection are proportional to the molar concentration of protons in the compounds. The concentration of each component can be easily calculated from the projection even though it is not exactly the same as a chromatogram.
This quantitative method can be used to quantify mixture components whether applied with regular solvents or with NMR chromatography solvents that enhance the differences in diffusivity between components.
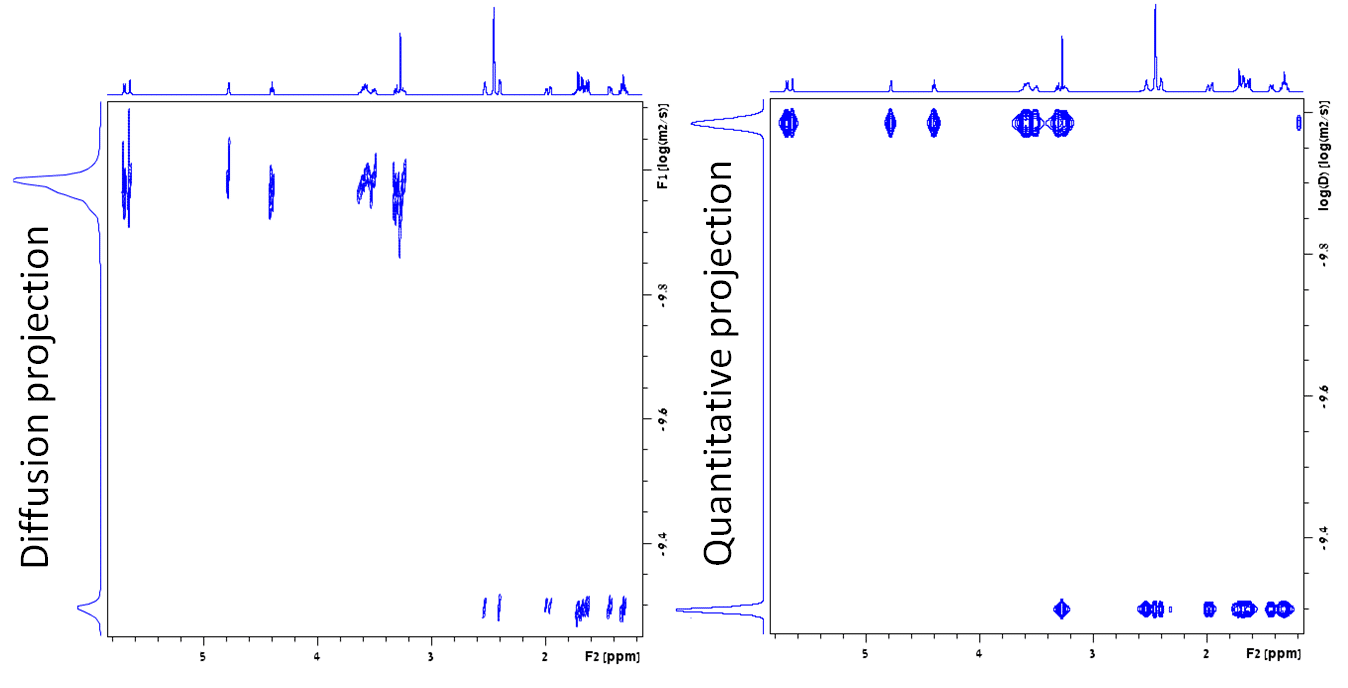
Figure. DOSY of a mixture of β-cyclodextrin and norcamphor in DMSO-d6. Note the difference in the diffusion projections: conventional processing on the left and quantitative processing on the right.
1 C. Barrère, P Thureau, A. Thévand, S. Viel, J. Magn. Reson, 216, 201-208 (2012).
Powered by Eventact EMS