
CHEMICAL SYNTHESIS OF PROTEINS WITH NON-STRATEGICALLY PLACED CYSTEINES USING SELENAZOLIDINE AND SELECTIVE DESELENIZATION
The development of native chemical ligation coupled with desulfurization has allowed ligation at several new ligation junctions. However, desulfurization also converts all cysteine residues in the protein sequence into alanine. Deselenization of selenocysteine, in contrast, selectively removes the selenol group to give alanine in the presence of unprotected cysteines. Here we shed more light onto the deselenization mechanism of selenocysteine to alanine and provide optimized conditions for the reaction. The deselenization can be accomplished in one minute under anaerobic conditions to give alanine. Under aerobic conditions (oxygen saturation), selenocysteine is converted into serine.
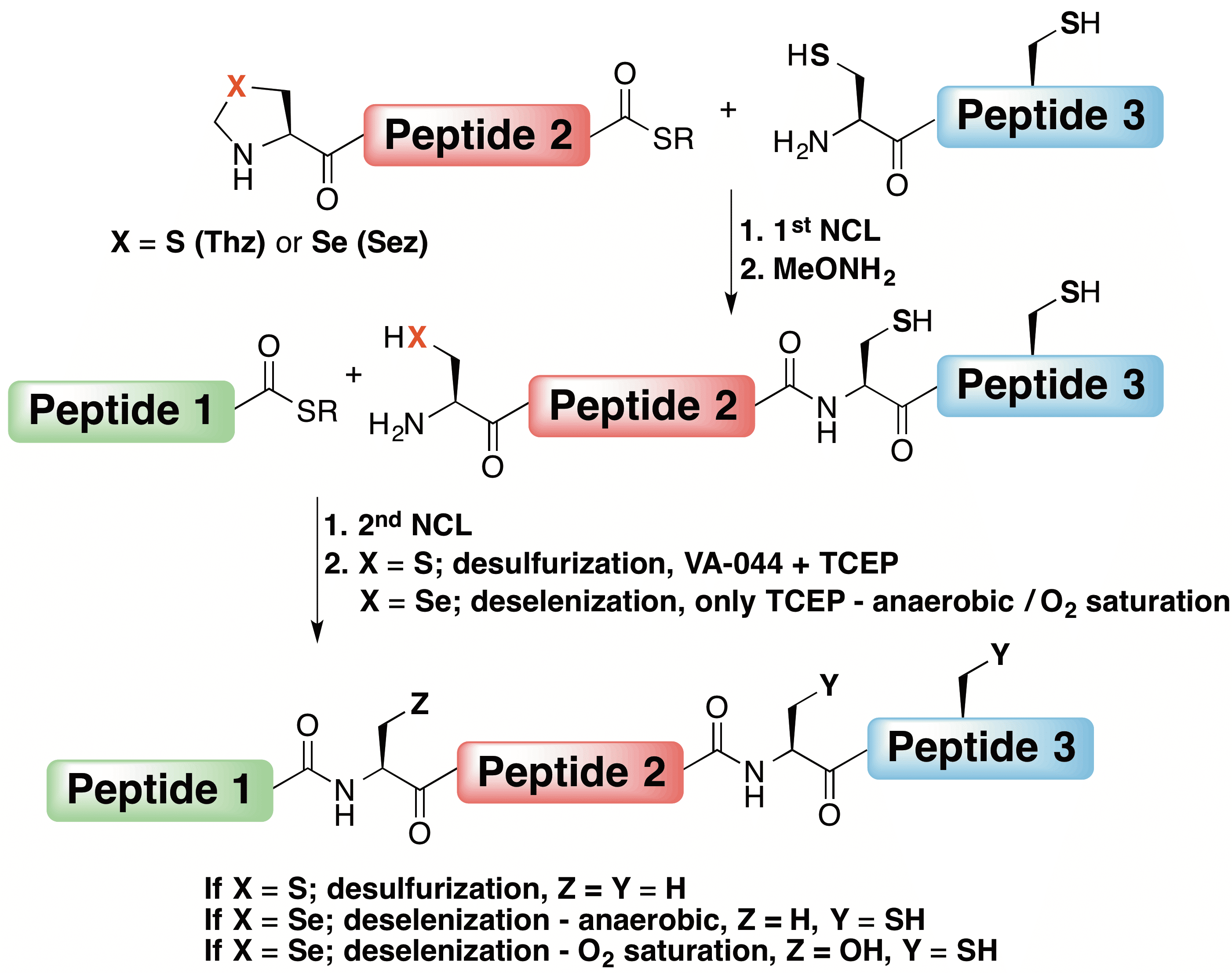
Although native chemical ligation has enabled the synthesis of hundreds of proteins, not all proteins are accessible through typical ligation conditions. The challenging protein, 125-residue human phosphohistidine phosphatase 1 (PHPT1), has three cysteines near the protein’s C-terminus, which are not strategically placed for ligation. Here, we report the first sequential native chemical ligation/deselenization reaction. PHPT1 was prepared from three unprotected peptide segments using two ligation reactions at cysteine and alanine junctions. Selenazolidine was utilized as a masked precursor for N-terminal selenocysteine in the middle segment, and, following ligations, deselenization provided the native alanine residue. This approach was used to synthesize both the wild-type PHPT1 and an analog in which the active-site histidine was substituted with the unnatural and isosteric amino acid β-thienyl-L-alanine. The activity of both proteins was studied and compared, providing insights into the enzyme’s active site.
Powered by Eventact EMS