
COT ISLAND INSIDE TWO FUSED CORROLES: PYRIDINE AXIALLY LIGATED FIVE COORDINATE DIIRON(III)CORROLE DIMER AND RELATED ELECTROCHEMISTRY
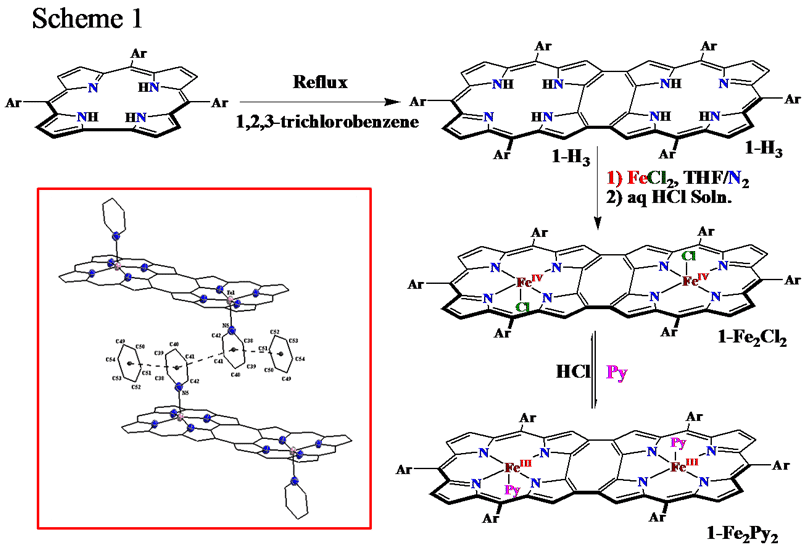
Direct synthesis of cyclooctatetraene (COT) bridged corrole dimer has been improved by minor modification of the reported procedure.1,2 Extensive Π-delocalization in the ligand produce extremely low energy band, that reach into infra red region, making this novel dimer very good candidate for bio-imaging studies. This prompts us to synthesize biologically relevant, easily oxidizable and reducible diiron complexes. Electrochemical and coordination properties of diiron-corrole dimer has been compared with previously reported monmeric analogs. The dimer exhibit unexpected preference for penta-coordination, whereas monomeric analog forms hexacoordinated complex.3,4
References:
(1) Tsuda, A.; Furuta, H.; Osuka, A. J. Am. Chem. Soc. 2001, 123, 10304.
(2) Barata, J. F. B.; Silva, A. M. G.; Neves, M. G. P. M. S.; Tome, A. C.; Silva, A. M. S.;
Cavaleiro, J. A. S. Tetrahedron Lett. 2006, 47, 8171.
(3) Simkhovich, L.; Mahammed, A.; Goldberg, I.; Gross, Z. Chem. Eur. J. 2001, 7, 1041.
(4) Simkhovich, L.; Galili, N.; Saltman, I.; Goldberg, I.; Gross, Z. Inorg.
Chem. 2000, 39, 2704-2705.
Powered by Eventact EMS