
TITANIUM MEDIATED SYNTHESIS OF TRIALLYLMETHANES. THE FIRST ALLYLATION OF ESTERS BY AN ALLYLSILANE
Allyl substituents are versatile groups that can be readily transformed to other functional groups by hydroboration, hydrocyanation, hydroformylation, epoxidation, aziridination or metathesis. Esters are potential starting materials for triallylmethanes, but unlike ketones or aldehydes, esters do not undergo Sakurai-Hosomi allylation with an allylsilane.
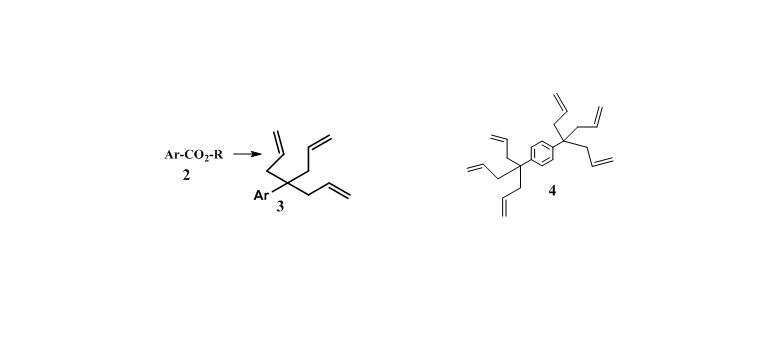
We report the first successful allylation of aromatic esters (e.g. 2) mediated by TiCl4 at room temperature. Other Lewis acids were not effective.
The triallylation process proceeds in a one-pot, domino reaction leading directly to quaternary triallylmethane derivatives 3, including dendrimer-like molecules 4.
Molecular modeling for the most efficient substrate (R: dimethoxyphenyl) reveals a bidentate Ti-complex, which improves the steric accessibility of the ester C=O to nucleophilic attack by the allylsilane.
Powered by Eventact EMS