
ION TRANSPORT IN A DENSE POLYMERIC MEMBRANE: A MOLECULAR DYNAMICS INVESTIGATION
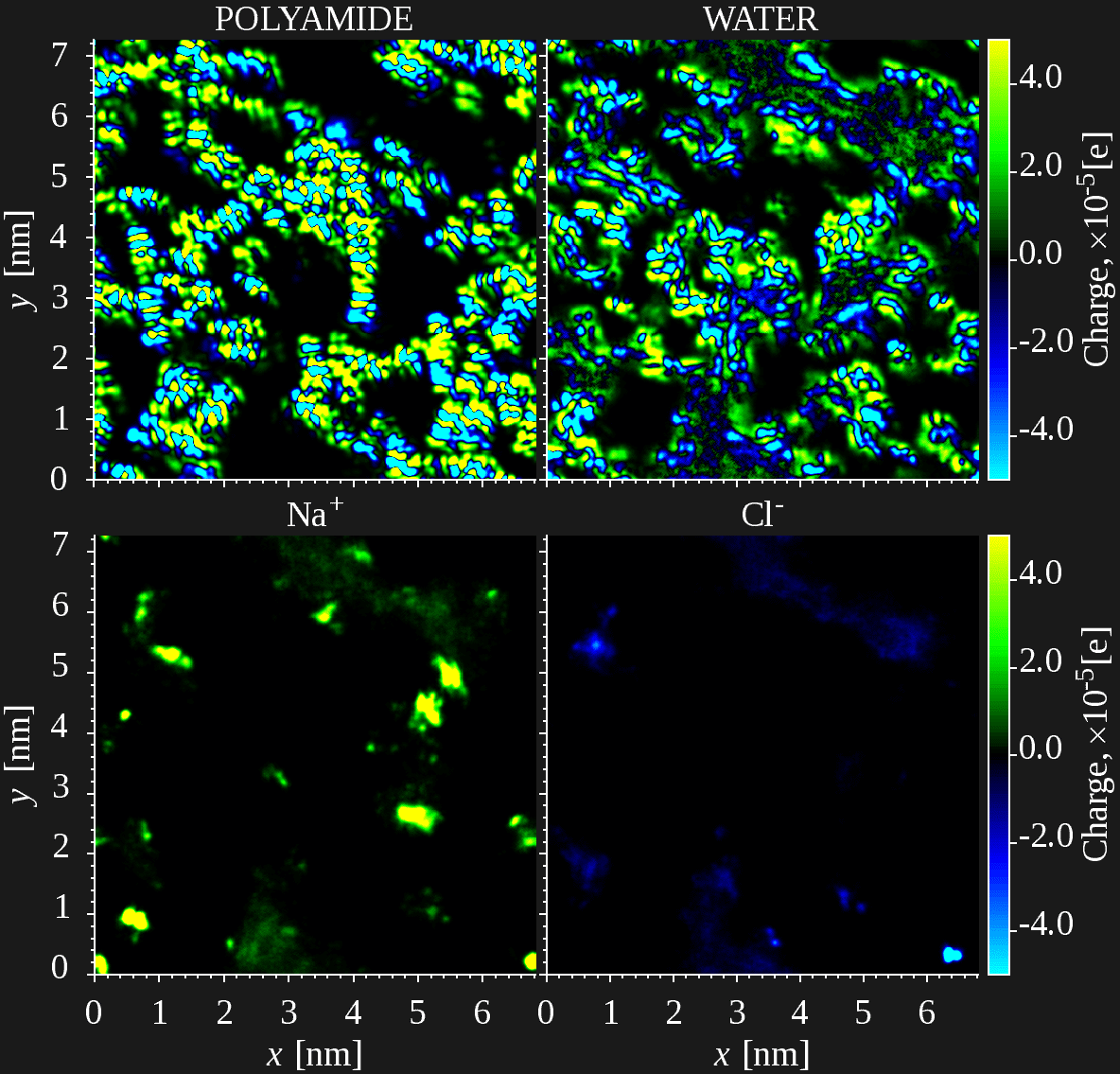
Mechanism of ion rejection in desalination membranes still puzzles scientists, despite decades of research and wide commercial exploitation. With the purpose to gain insights into the mechanisms of ion uptake and permeation in desalination membranes, MD investigation of a model polyamide membrane was carried out on a relatively large model system (45K atoms) closely matching real membranes in terms of chemistry and water permeability. Simulations demonstrate that the mechanism of ion uptake distinctly differs from mean-field approaches that assume a smeared Donnan potential and Born-like self-energy of the ions in the membrane phase. Ion sorption on charged sites in the membrane phase appears to be highly localized (see Figure), due to electrostatic forces dominating over translational entropy. Moreover, sorption on partial atomic charges becomes possible as well, which greatly enhances salt (co-ion) uptake and weakens the effect of fixed charges on salt exclusion. This explains high ion uptake measured in polyamide membranes, seemingly inconsistent with low slat permeability, and puzzling dependence of ion sorption and permeation observed at low salt concentrations. On the other hand, present simulations greatly overestimate salt permeability, highlighting its great sensitivity to the polymer microstructure and connectivity.
Powered by Eventact EMS