
INTEGRIN INACTIVATION BY ORGANOTELLURANES: POTENTIAL ROLE IN THEIR ANTI-METASTATIC ACTIVITY
2The Mina & Everard Goodman Faculty of Life Sciences, C.A.I.R. Institute, The Safdiè AIDS and Immunology Research Centre, Bar-Ilan University
3Department of Biological Regulation, Faculty of Biology, Weizmann Institute of Science
The investigation of therapeutic activities of tellurium compounds is still in its early stages, despite the relative abundance of tellurium in the human body. A few hypervalent organic Te(IV) compounds (organotelluranes), differing in their labile ligand moieties, were found by us to exhibit anti-integrin activities in vitro and to demonstrate anti-metastatic properties in vivo. The synthetic organotelluranes are reactive towards L-cysteine as a thiol model compound. They inhibit integrin functions as adhesion, migration and metalloproteinase secretion mediation in B16F10 murine melanoma cells. In comparison, a reduced derivative, which contains no labile ligands, inhibits integrins to a significantly lower extent, pointing to the highly important role of the labile ligand moieties, bound to the Te(IV) atom. Moreover, incubation with the impermeable mercurial sulfhydryl reagent p-chloromercuribenzene sulfonate (pCMBS) did not affect the migratory activity of B16F10 cells, as compared with organotellurane 1, suggesting that oxidation of cysteines and the formation of disulfide bonds are necessary for inhibiting integrins. Organotellurane 1 is further established as a potential non-toxic and anti-metastatic Te(IV) based drug for the treatment of metastatic melanoma, inhibiting the formation of liver metastases in C57Bl/6 mice. Our results extend the current knowledge on the reactivity and mechanism of organotelluranes with different labile ligands and highlight their clinical potential.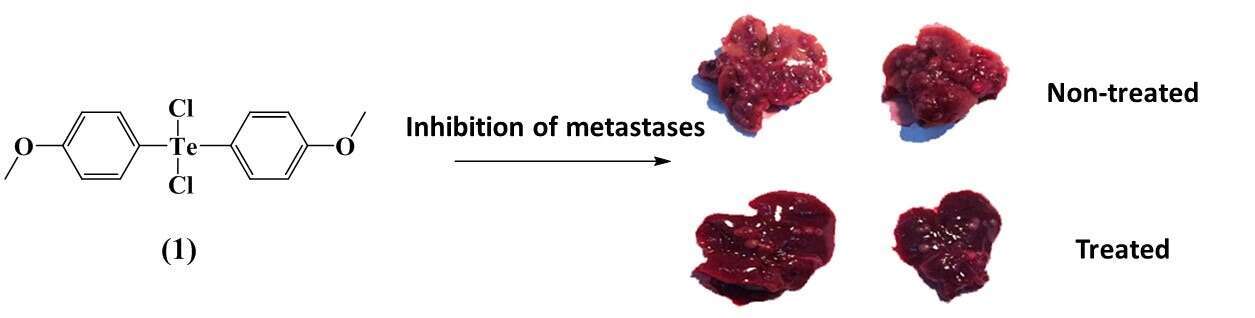
Powered by Eventact EMS