
ZIRCONIA COATING ON NICKEL-RICH Li[Ni0.8Mn0.1Co0.1]O2 CATHODE MATERIAL VIA SONOCHEMICAL MEANS AND CLASSIC PRECIPITATION AND THE HIGH TEMPERATURE TRANSITION INTO A SURFACE DOPING
With Lithium ion batteries (LIB) becoming the power source of choice in the portable market cell manufactures are pushing to place LIB in the crosshair of automobile companies. The family of Li[NiCoMn]O2 (LiNCM) is expected to play a vital role in the development of electric vehicles (EV) and a number of compositions have been synthesized and partially commercialized.
The increase in capacity is driven by the increase in nickel content in LiNCM which in turn increases surface reactivity and spinel formation of the bulk material, both of which translate to a capacity fading. Surface coatings can retard parasitic reactions of the active material with the electrolyte solution but fail to influence bulk properties. One convenient way of doing so is by doping the material with metal ions such as Mg2+, Al3+ and Zr4+. We will present an easy way of to perform a post synthesis doping of nickel rich LiNCM with Zr4+ via a simple heat treatment. The zirconia coating can be transformed into a zirconium doping which as was confirmed via XPS (fig. 1 a). Furthermore, the cycling behavior of the material is enhanced compared to its untreated precursor (fig. 1 b). The synthesis and in-depth characterization will be presented to elucidate the why and how of the increase in performance.
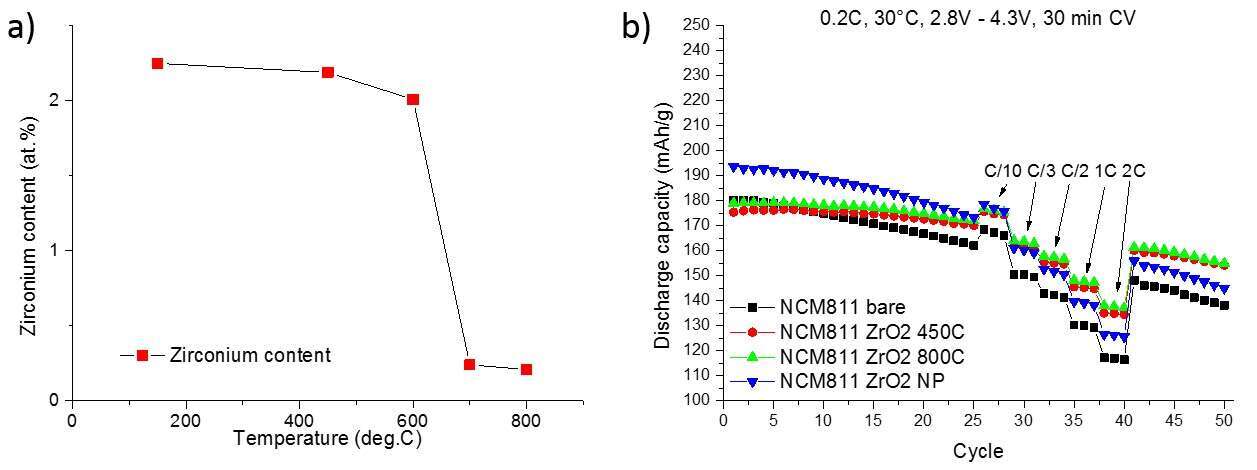
Figure 1 a) Zirconium content detected by XPS of coatings annealed at different temperatures, showing the decrease in surface zirconium
Figure 1 b) Cycling behavior of treated material in comparison to the bare material at 30ºC and 0.2 C
Powered by Eventact EMS