
EXPLORING THE EFFECTS OF GLYCOSYLATION AND ETHERIFICATION OF THE SIDE CHAINS OF THE ANTI-CANCER DRUG MITOXANTRONE
2Department of Neurobiology, Tel Aviv University
Mitoxantrone (MTX) is a chemotherapeutic drug that is used for the treatment of several cancers including breast tumors, acute myelogenous leukemia, non-Hodgkin`s lymphoma and prostate cancer. The clinical utility of MTX as antitumor agent is compromised by its dose-limiting cardiotoxicitiy. This side effect occurs in approximately one fifth of the patients treated with the drug and limits its lifetime maximum dose. There is evidence that one of the causes for the enhanced cardiotoxicitiy is the formation of MTX metabolites.
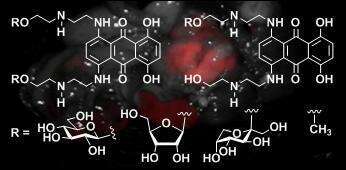
In this study we synthesized a collection of symmetric and asymmetric MTX analogues in which the primary alcohol of one or both side chains was modified by O-glycosylation or methyl etherification. We studied the impact of these modifications on target DNA affinity, cytotoxic activity, and metabolism and evaluated the potential of these chemical modifications to lead to the development of novel MTX-derived anticancer drugs.
Our study demonstrates that derivatization of MTX side chains primary alcohols may result in a significant improvement in DNA affinity and lower susceptibility to the formation of potentially toxic metabolites. Relatively small changes of the primary alcohol in each of the MTX side chains resulted in a profound effect on the pharmacokinetic properties.
The results presented in this study suggest that MTX analogues with modified side chains primary alcohols offer a promising direction for the development of new antitumor agents.
Powered by Eventact EMS