
WERNER COMPLEXES: A NEW CLASS OF CHIRAL HYDROGEN BOND DONOR CATALYSTS FOR ENANTIOSELECTIVE ORGANIC REACTIONS
Salts of the chiral tris(ethylenediamine)-substituted octahedral trication [Co(en)3]3+, and related species, have played important historical roles in the development of inorganic chemistry and stereochemistry.1,2 As Werner described in 1912, the two enantiomers, commonly designated L and D, can be separated by crystallization of the diastereomeric tartrate salts.2 However, despite the low cost and ready availability of the building blocks, there have been no applications in enantioselective organic synthesis.
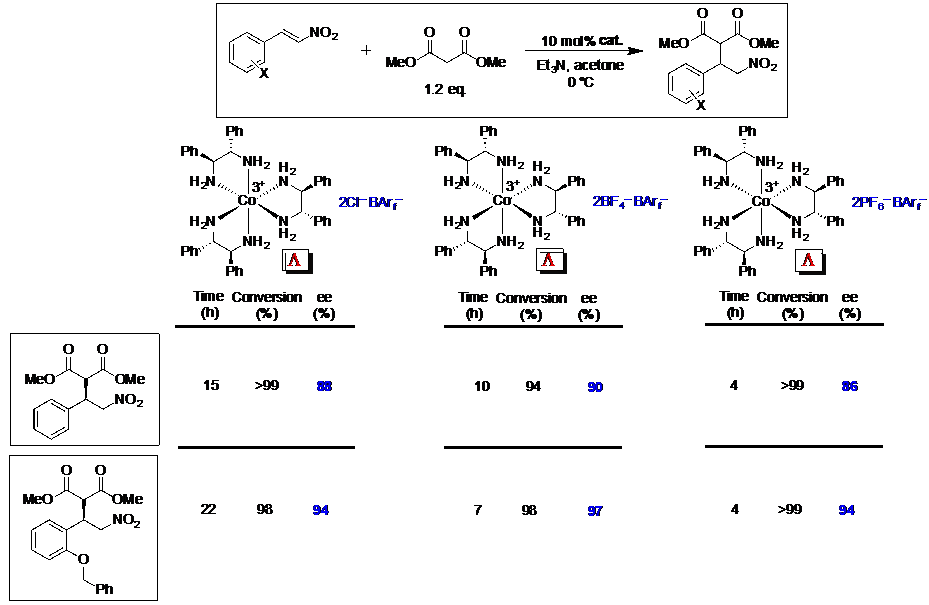
We have found that [Co(en)3]3+ and related cations can be rendered soluble in organic solvents by using lipophilic anions such as "BArf–".3-5 Suitably functionalized derivatives act as highly enantioselective catalysts for a variety of carbon-carbon bond forming reactions. The mechanisms involve outer sphere activation of the electrophile by hydrogen bonding to the NH moieties. Other types of metal-containing chiral hydrogen bond donors are also effective, including a chelate of the CpRuL fragment.6,7
1 Kauffman, G. B. Coord. Chem. Rev. 1974, 12, 105.
2 Werner, A. Chem. Ber. 1911, 44, 1887 and 1912, 45, 121.
3 Ganzmann, C.; Gladysz, J. A. Chem. Eur. J. 2008, 14, 5397.
4 Lewis, K. G.; Ghosh, S. K.; Bhuvanesh, N.; Gladysz, J. A. ACS Central Science 2015, 1, 50.
5 Ghosh, S. K.; Ojeda, A. S.; Guerrero-Leal, J.; Bhuvanesh, N.; Gladysz, J. A. Inorg. Chem. 2013, 52, 9369.
6 Thomas, C.; Gladysz, J. A. ACS Catalysis 2014, 5, 1134.
7 (a) Scherer, A.; Mukherjee, T.; Hampel, F.; Gladysz, J. A. Organometallics 2014, 33, 6709. (b) Mukherjee, T.; Ganzmann, C.; Bhuvanesh, N.; Gladysz, J. A. Organometallics 2014, 33, 6723.
Powered by Eventact EMS