
NEW, EFFICIENT, GENERAL AND METAL-FREE BROMODECARBOXYLATION
It’s difficult to overstate the importance of organobromine compounds for laboratory synthesis and chemical industries. They are routinely used in a vast array of chemical transformations as building blocks for pharmaceuticals, agrochemicals and dyes.[i] Among organic halides, bromine compounds are one of the most popular and widely employed. They are much more reactive than organochlorine compounds, as well as cheaper and more stable than the corresponding alkyliodides.
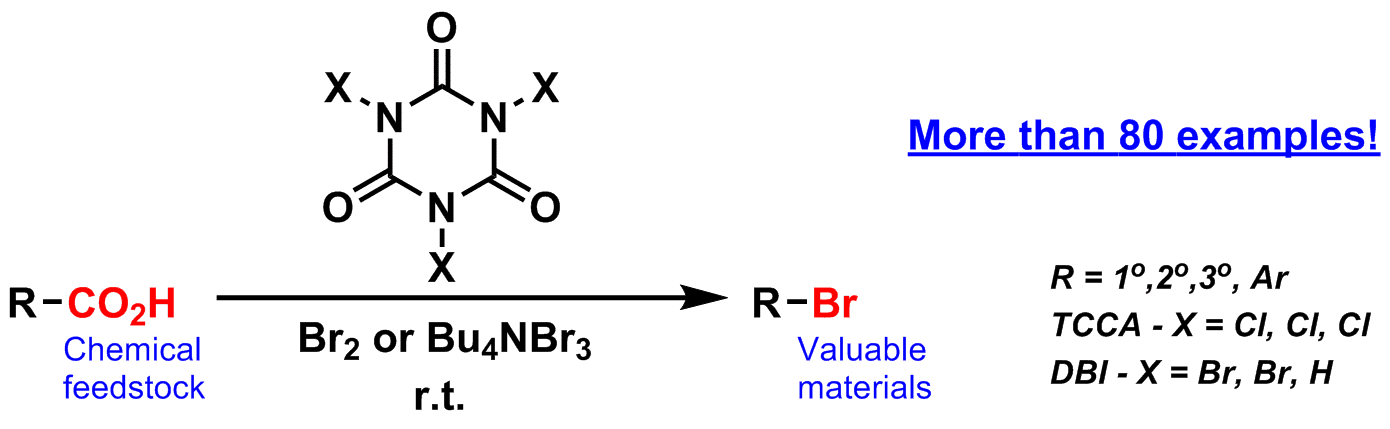
Bromodecarboxylation is one of the most selective and attractive methods for the synthesis of brominated compounds. The synthesis of alkyl bromides from carboxylic acids has been known since 1939, with many different methods developed.[ii] Although extensively deployed in research laboratories, they have found little foothold in industry. The reason is that all of existing methods are complicated, require expensive, oxygen and moisture sensitive[iii], toxic[iv] and highly-regulated chemicals (for instance by the FDA).
Here we present a new, extremely simple, efficient and metal free bromodecarboxylation procedure. Our methodology uses chemical reagents generally recognized as benign and recyclable with our protocols, and tolerant of ambient conditions which are inimical to traditional reagents employed in this type of synthesis. The reaction is facile, and usually no complex product purification is required. The method is general: primary, secondary, tertiary alkyl bromides as well as aryl bromides can be prepared in high yields from corresponding carboxylic acids.
[i] Bromine Organic Compounds, “Kirk-Othmer Encyclopedia of Chemical Technology”, John Wiley and Sons, 2014.
[ii] Hassner A., Namboothiri, I., “Organic Syntheses Based on Name Reactions - A Practical Guide to 750 Transformations”, Elsevier, 2012, p. 232.
[iii] Johnson, R. G., Ingham, R. K. Chem. Rev. 1956, 56, 219-269.
[iv] Cristol, S.J., Firth, W.C., J. Org. Chem., 1961, 26, 280.
Powered by Eventact EMS