
DENDRITIC LIGANDS FOR ACTINIDE AND LANTHANIDE COORDINATION
Complexes of actinide metal ions with specific ligands are extensively used for many applications in the field of chelation therapy. The most frequently used ligands for the targeted α-therapy are poly-bidentate and employ hard basic moieties, such as HOPO (hydroxy-pyridinone).
Encouraged by the high affinity of the 1,2-HOPO ligands towards actinides and, accordingly, their impressive potential to be used as sequestering agents for such metal ions, we decided to develop a series of new chelators for actinides, which will be used for therapeutic applications (e.g. targeted α-therapy and actinide de-corporation).
These new chelators are based on a dendritic backbone and carry peripheral 6-carboxamide-1,2-HOPO chelating units (Figure 1). The dendritic architecture produces, in the case of the second generation G2 dendron, a tetra-HOPO arrangement essential for a successful sequestering of actinide metal ions, whose preferred coordination numbers are 6 or 8. The carboxamide function of the chelators assists in adjusting the pKa of the free ligand to form stable complexes under physiological conditions and serves as a connector to the backbone. The non-dendritic analogue (G0) and the first generation dendron (G1) serve as models for the developing of the methodology for the synthesis of the higher generation target.
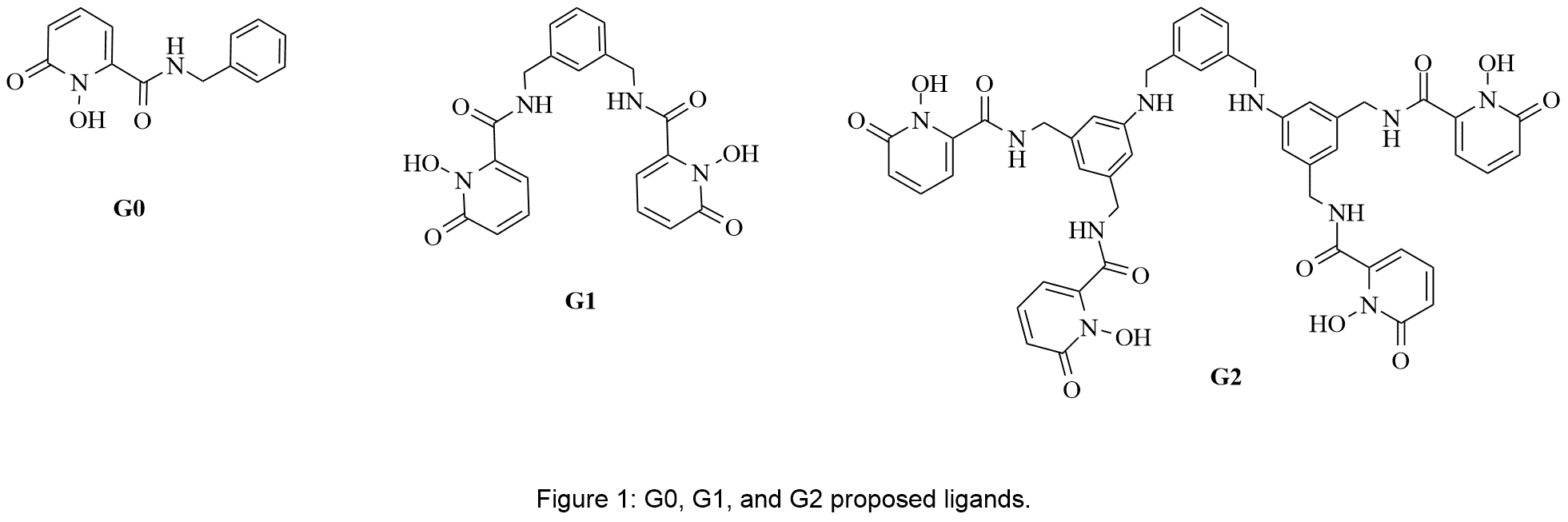
Powered by Eventact EMS