
ORGANOCATALYST-METAL CO-CATALYSIS OF THE BAYLIS-HILLMAN REACTION
The Baylis-Hillman reaction is an efficient, useful carbon-carbon bond-forming reaction, which acquired high synthetic popularity due to its operational simplicity and the enormous application potential of the Baylis-Hillman products. Recently, it has been observed in our group that, while N-alkylimidazole-based polymer-supported catalyst exhibit outstanding chemoselectivity in this reaction, their homogeneous analogues promote the reaction with low selectivity, i.e. forming multiple byproducts along with the Baylis-Hillman adduct.
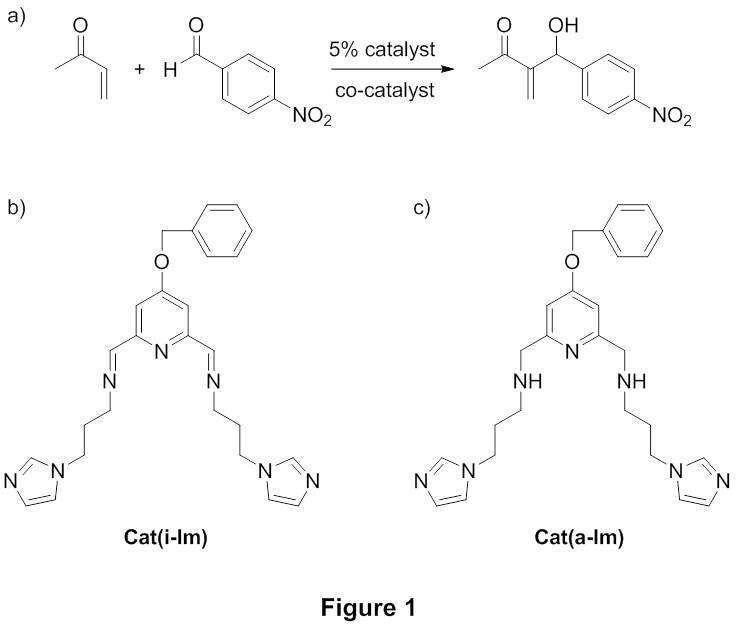
The combination of organocatalysis and transition metals is an emerging field in organic chemistry. It has been reported that transition metals, functioning as Lewis acids, can be added as co-catalysts to organocatalytic systems. In attempt to implement this approach for the Baylis-Hillman reaction (Figure 1a), we synthesized bifunctional catalysts (Figure 1b and c), which include N-alkylimidazole units as catalysts and a pyridine-based chelate as a ligating site for the transition metal as a co-catalyst.
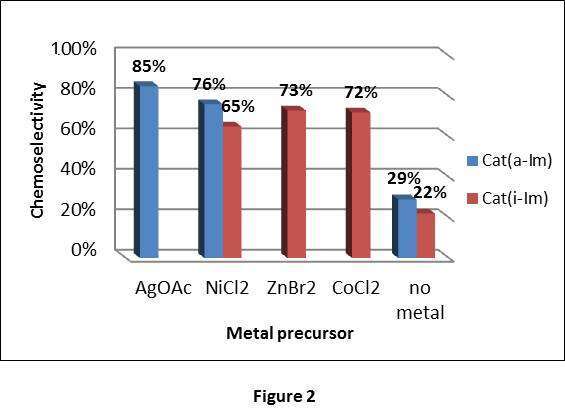
When various metal additives were added to the catalytic system, some of the metals caused a dramatic improvement in the chemoselectivity of the catalysis (Figure 2), reaching above 70% selectivity (versus 20-30% without the metal additive).
This poster describes the effect of different parameters, such as the nature of the metal, its concentration, and the structure of the new catalytic systems, on the Baylis-Hillman reaction.
Powered by Eventact EMS