
SYNTHESIS AND CHARACTERIZATION OF METAL-PHOSPHIDO MACROCYCLES OF ZINC AND MERCURY WITH A NOVEL M4P4 CAGE
A macrocyclic metal-phosphido cages can potentially serve as a new class of crown molecules heaving Lewis acidic metals and Lewis basic phosphido groups which are capable to bind simultaneously cation and anion pairs, respectively.
We now report a facial synthesis and characterization of solvent-free functional (FSi(R2)PM)4 macrocyclic cages where M=Zn, Hg, R=tBu2MeSi. Thus, the reaction of phosposilane 1 with metal bisamides (2) produces the thermally stable tetra-metallocyclic complexes 3 (M=Zn) and 4 (M=Hg). 3 is the first structurally characterized zinc primary phosphide cycle in which Zn is two-coordinated, linear (P-Zn-P: 178°), and covalently bonded to two phosphorus atoms (relatively short bond Zn-P: 2.26Å) leaving the phosphorus lone pair available for additional coordination.
We are currently studying the coordination abilities of these macrocyclic metallo-cages towards ion pairs and small molecules. 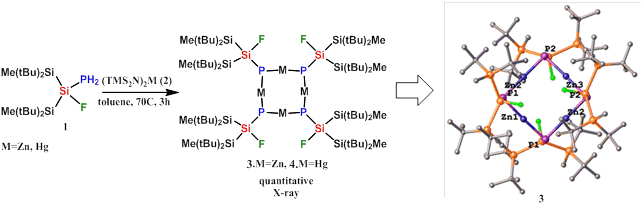
Powered by Eventact EMS