
NOVEL CLASS OF LEWIS ACID BASED ON CATIONIC NITROGEN.
SYNTHESIS OF UNPRECEDENTED ALL-SATURATED N-N-N (BIS-AMINO)AMINE COMPOUNDS
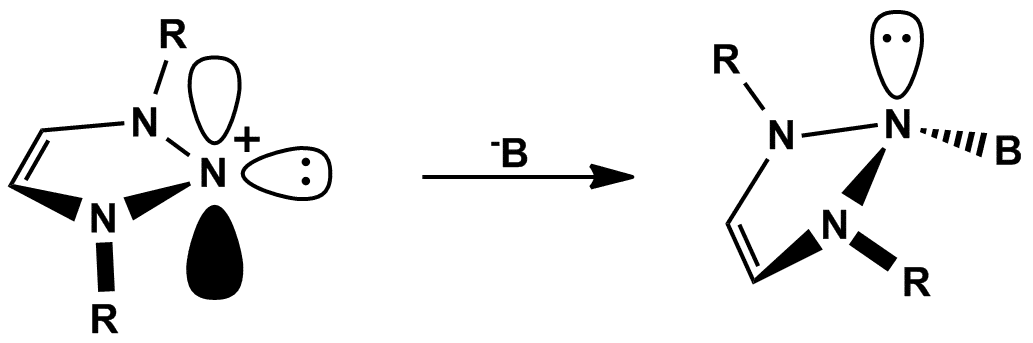
N-heterocyclic nitrenium ions are the nitrogen-derived cationic analogues of N-heterocyclic carbenes. Unlike N-heterocyclic carbenes and their analogues, these species have been known for many years as stable and coordinatively-inert molecules. Recently, our group reported the preparation of several N-heterocyclic nitrenium ions and demonstrated coordination of these species to various transition metal centers (such as Rh, Ru and Pt), forming mono- , di- and even tri-cationic complexes.[i]
Here we present, for the first time, the novel function of nitrenium species. The availability of vacant pp-orbital of nitrenium species suggests that it possess Lewis acid character and might be involved in s-type interactions with various Lewis bases. As such, we demonstrate that nitrenium species afford a novel type of Lewis acid-base pairs.[ii] Moreover, based on this reactivity, we have discovered a new type of organic compounds, bearing all-saturated sp3-hybridized three consecutive nitrogen atoms. Interestingly, these unprecedented N-N-N compounds are sufficiently stable and can be crystallized. In light of chemistry of Lewis acid-base pairs based on carbenes and their analogues and frustrated Lewis pairs, we hope that these new pairs will expand the field of metal-free activation of small molecules.
[i] (a) Tulchinsky, Y.; Iron, M. A.; Botoshansky, M.; Gandelman, M. Nature Chem. 2011, 3, 525. (b) Tulchinsky, Y.; Kozuch, S.; Saha, P.; Botoshansky, M.; Shimon, L.; Gandelman, M. Chem. Sci. 2014, 5, 1305. (c) Tulchinsky, Y., Kozuch, S., Saha, P., Mauda, A., Nisnevich, G., Botoshansky, M., Shimon, L., Gandelman, M. Chem. Eur. J. 2015, 21, 1-13.
[ii] Pogoreltsev, A., Tulchinsky, Y., Fridman, N., Botoshansky, M., Gandelman, M. Manuscript in preparation.
Powered by Eventact EMS