
TOWARDS NEW CHAMELEON CATALYSTS
2Department of Natural Sciences, The Open University of Israel
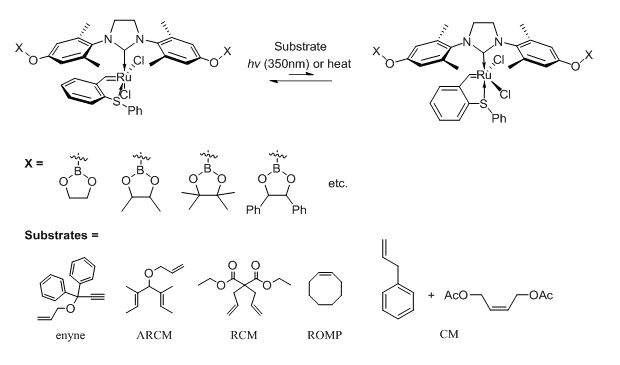
Recently, our group developed and studied catalytic chameleon dendrimers.1 Chameleon dendrimers are catalysts that can change their selectivity depending on their chemical surroundings. For example, porphyrin core dendrimers displayed different selectivity towards epoxidation reactions of olefins depending on the type of interchangeable boronic ester termini. The exploration of this concept in olefin metathesis catalysts may provide selective pathways for one of the most powerful tools in synthetic organic and polymer chemistry developed during the last decades.2
Herein, we report our preliminary results on the synthesis and activity of photo- and thermal- activated latent sulfur chelated ruthenium complexes3 bearing dendritic N-Heterocyclic Carbene (NHC) ligands with boronic ester appendages as a new type of chameleon catalysts. By transesterification of the boronic ester termini of different catalysts (generations) with diols we investigate the effect on selectivity for different types of olefin metathesis reactions (RCM, CM, ROMP, etc.) and substrates.
[1] Shema-Mizrachi, M.; Pavan, G.M.; Levin, E.; Danani, A.; Lemcoff, N.G., Catalytic Chameleon Dendrimers, J. Am. Chem. Soc., 2011, 133, 14359-14367.
[2] Diesendruck, C.E.; Tzur, E. and Lemcoff, N.G, Eur. J. Inorg. Chem. (Feature Cover Article), 2009, 28, 4185-4203.
[3](a) A. Ben-Asuly, E. Tzur, C. E. Diesendruck, M. Sigalov, I. Goldberg, N. G. Lemcoff, Organometallics, 2008, 27, 811. (b) Ben-Asuly, A.; Aharoni, A.; Diesendruck, C.E.; Vidavsky, Y.; Goldberg, I.; Straub, B. F. and Lemcoff, N.G., Organometallics, 2009, 28, 4652-4655.
Powered by Eventact EMS