
ELECTROCHEMICAL OXIDATION OF BISAMIDES IN METHANOL
Recently we found1-2 that methoxylation of `cyclic amides` occurs at the a and a` positions to `N` upon anodic oxidation in methanol, at constant current Major products include a-methoxy and a,a`-dimethoxy `cyclic amides` (Scheme 1):
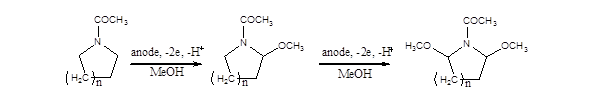
Scheme 1. Methoxylation of cyclic amides (n= 0, 1, 2)
In the present research we have investigated a series of bisamides (Scheme 2) in methanol at various anodes, applying a current density of 20 mA/cm2. Various methhoxylated products were formed and the selectivity was found to be dependent on both the type of substrate and number of methylene groups in the spacer between the two amide functinalities.
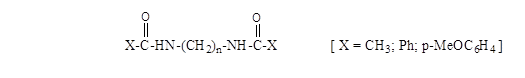
Scheme 2. Types of bisamides (n = 2, 3, 4)
References:
[1] T. Golub, J.Y. Becker, "Anodic oxidation of N-acylazacycloalkanes in methanol", J. Electrochem. Soc., 160 (2013) G3123.
[2] T. Golub, J.Y. Becker, "The effect of N-acyl and N-sulfonyl groups on anodic methoxylation of piperidine derivatives", Electrochim. Acta 173 (2015) 408.
Powered by Eventact EMS