
DESIGN AND SYNTHESIS OF STABLE α-HYDROGEN NITROXIDES
2Department of Chemical Research Support, Weizmann Institute of Science
The nature and use of nitroxide compounds as stable radicals and as reactive reagents and catalysts for redox reactions gives them a unique place in organic chemistry.[1],[2] Designed nitroxide compounds have been used as radical trapping reagents, catalysts for oxidation of alcohols into carbonyl compounds and allylic transposition reactions. They also have applications in biology and medicine.
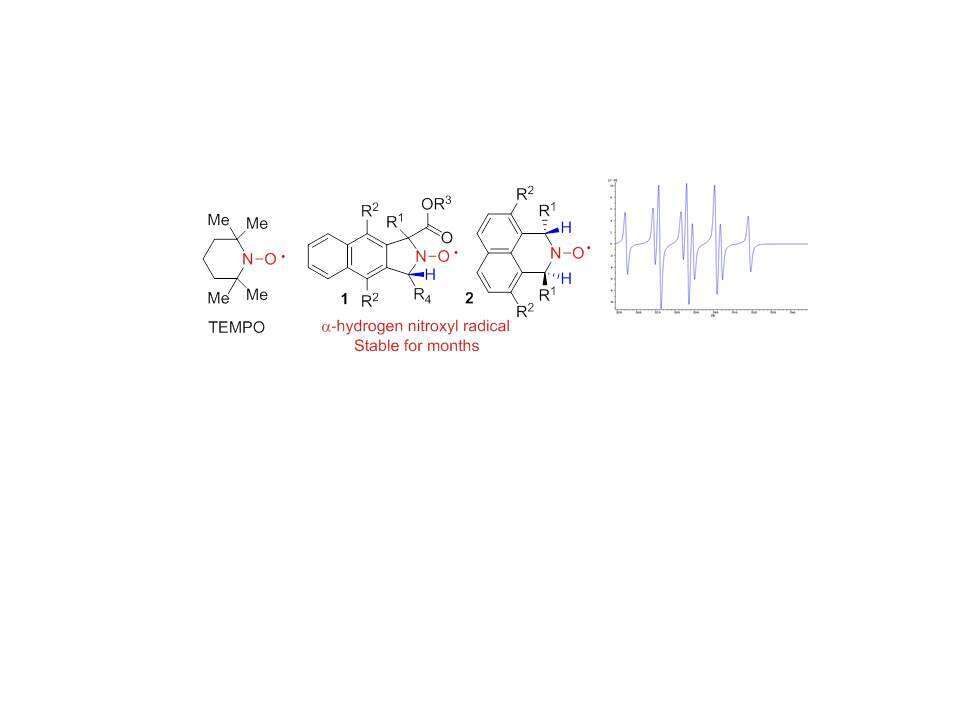
TEMPO, is one of the most efficient and widely used nitroxides exemplifies the archetypal nitroxide structure with its four flanking methyl groups. Unfortunately, the design of nitroxyl radicals with a more diverse set of substituents constitutes a significant synthetic challenge. A logical way to diminish the steric congestion is to replace an α-alkyl substituent with a hydrogen. However, α-hydrogen nitroxyl radicals are extremely unstable and disproportionate to form nitrones and hydroxylamines.
Recently, we developed a new concept for designing stable α-hydrogen nitroxyl radicals two new families of nitroxyl radicals namely IAPNO 1 and IINO 2.[3]-4
In this poster we report a significant number of new radicals of these families and report on the structural requirements for achieving nitroxide radical stability.
[1] Tebben, L.; Studer, A. Angewandte Chemie International Edition 2011, 50, 5034.
[2] Bobbitt, J. M.; BrüCkner, C.; Merbouh, N. In Organic Reactions; John Wiley & Sons, Inc.: 2009.
[3] Michal Amar, Sukanta Bar, Mark A. Iron, Hila Toledo, Boris Tumanskii, Linda J. W. Shimon, Mark Botoshansky, Natalia Fridman, and Alex M. Szpilman Nature Communications, 2015, 6, 6070.
[4] H. Toledo, M. Amar, S. Bar, M. A. Iron, N. Fridman, B. Tumanskii, L. J. W. Shimon, M. Botoshansky, A. M. Szpilman, Organic & Biomolecular Chemistry 2015, 13, 10726-10733.
Powered by Eventact EMS