
CONSTRAINING BACTERIAL SIGNALING
One of the mechanisms that bacteria use to communicate with each other is termed `quorum sensing` (QS). QS is based on the secretion and sensing of small molecules that, only when reaching a certain threshold concentration, trigger signaling cascades inside the cells that will eventually result in population wide simultaneous gene expression. This mechanism enables a bacterial population to benefit from collaborative behavior, such as the formation of a bacterial biofilm.
One of the Gram negative bacteria effectively uses QS to establish infections in human hosts is Pseudomonas aeruginosa, and its QS regulon is partly known. The signaling cascade in the cell is triggered first by 3-oxo-dodecanoyl homoserine lactone (3-oxo-C12-HSL, or C12) while other known signals (such as butyryl HSL (C4) and PQS) also play important roles.
In this study we decided to focus on the primary signaling molecule C12 and to modulate the affinity of analogs for the target receptor LasR through constrainment of its flexible lipid chain. By fixing the ligand in the form that it would adopt ideally inside LasR (as observed by x-ray crystallography) we expect a strong increase in its affinity for LasR. We will examine the effects of such constraints on bacterial behavior.
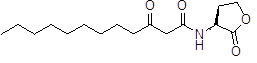
N-(3-oxododecanoyl)homoserine lactone
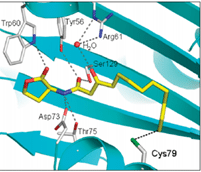
C12 inside the binding pocket of LasR
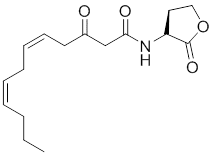 Target molecule
Target molecule
Powered by Eventact EMS