
SYNTHESIS OF CF3-SUBSTITUTED COMPOUNDS BY HIYAMA CROSS-COUPLING REACTIONS OF SECONDARY CF3-ELECTOPHILES
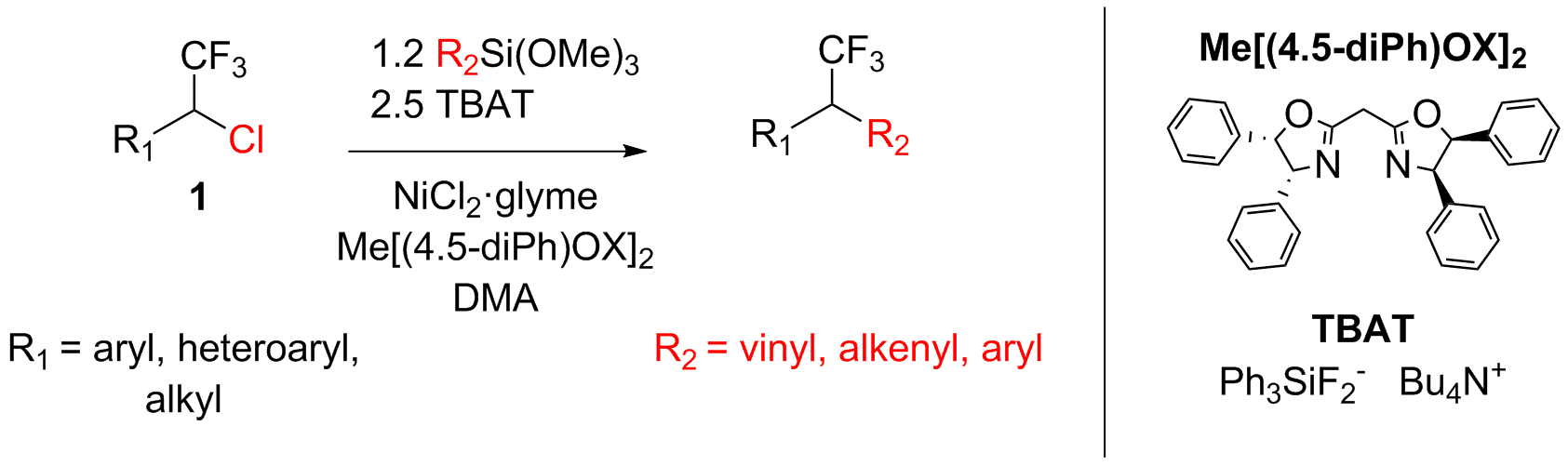
Fluorine-containing organic compounds are of high interest to chemical community, as introduction of fluorine could drastically change properties of molecule (i.e. polarity, stability, reactivity, etc.). During last years significant number of methods for incorporation of fluorine and fluorinated moieties was developed. Among them cross-coupling reactions stand out due to theirs mild reaction conditions and large functional group tolerance. While most of this methods are convenient for incorporation of CF3-group into aryl ring1, very few of them suitable for the synthesis of structures containing sp3-Rf bonds2,3 (Rf - perfluoroalkyl).
Recently it was shown that 1-fluoro-1-halo-alkanes undergo efficient Suzuki cross-coupling reaction to give chiral tertiary fluorides4. We extend this idea on perfluorinated derivatives to give 1-chloro-1-aryl-2,2,2-trifluoroethanes, 1, as the starting backbone for cross-coupling reaction.
It was found that 1 undergoes efficient nickel-catalyzed Hiyama cross-coupling reaction with vinyl(trimethoxy)silane in good to excellent yields while being compatible with wide range of different functional group-substituted aryls. Reaction could also be extended on heteroaryl moieties. Moreover, reaction is not limited to activated electrophiles: 1-alkyl-1-chloro-2,2,2-trifluoroethanes also reacting at standard conditions with good yields. Also, substituted vinyl(trimethoxy)silanes, such as (E)-1-(trimethoxysilyl)octene-1 and (E)-1-(trimethoxysilyl)-2-phenylethene, could be used. Besides that, different aryl(trimethoxy)silanes also could be utilized in this reaction.
- Alonso, E. Martinez de Marigorta, G. Rubiales, F. Palacios; Chem. Rev. 2015, 115, 1847;
- Liang, G.C. Fu; Angew. Chem. Int. Ed., 2015, 54, 9047;
- Li, Z. Feng, Z.-X. Jian, X. Zhang; Org. Lett.,2015, 17 (22), 5570;
- Jiang, S. Sakthivel, K. Kulbitski, G. Nisnevich, M. Gandelman; J. Am. Chem. Soc.,2014, 136 (27), 9548;
Powered by Eventact EMS