
"QUORUM SENSING IN TIMES OF CHOLERA“ - TARGETING THE BACTERIAL VIRULENCE REGULATOR IN V. CHOLERAE WITH ELECTROPHILIC PROBES
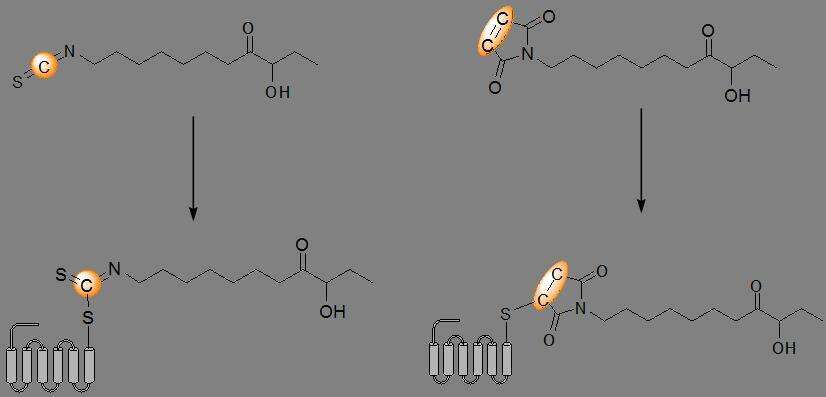
Antibiotic-resistant pathogens have become a rising and troubling phenomenon in recent years. A potential solution may come through fighting infections by targeting virulence mechanisms, as opposed to killing the bacteria, thereby avoiding the strong selective pressures that encourage antibiotic-resistant strains to develop. One strategy to achieve this is to disrupt quorum sensing (QS), the mechanism by which bacteria communicate with one another via small signaling molecules in order to coordinate their behavior, often including pathogenic behavior. For example, in Vibrio cholerae, a Gram-negative pathogen estimated to cause 100,000–120,000 deaths each year, QS mediates biofilm formation and the production of virulence factors through CAI-1, an α-hydroxyl ketone signaling molecule. We have synthesized CAI-1 analogues containing electrophilic moieties with the aim of covalently binding them to a specific cysteine residue in the CAI-1 receptor, CqsS. The covalent bond is estimated to lead to a conformational change in the receptor and thus change its biological activity. Once the probe is covalently bound, it will be possible to selectively label the modified CAI-1 with a fluorescent aminooxy-tag as previously reported by our group. The advantage of this method is that the binding of the probe to the receptor and the labeling are two separate reactions, thereby avoiding a decrease in affinity of the probe to the receptor due to a bulky tag. Moreover, covalently binding a CAI-1 analogue to its receptor can be later used forlive cell imaging and further investigation of the QS system in V. cholerae, opening the way for innovative, new ways to treat the infection.
References:
Ng WL, Wei Y, Perez LJ, Cong J, Long T, Koch M, Semmelhack MF, Wingreen NS, Bassler Bl (2010) PNAS 109(12): 5575
Rayo J, Amara N, Krief P, Meijler MM, (2011) JACS 133: 7469
Powered by Eventact EMS