
UNRAVELING INTERKINGDOM INFORMATION FLOW BETWEEN BACTERIA AND HUMANS
2National Institute of Biotechnology in the Negev, Ben-Gurion University of the Negev, Beer-Sheva
We investigate fundamental questions in the realm of interkingdom communication, focusing on the interactions between bacteria and higher organisms. Recently, exciting findings have demonstrated that molecules involved in quorum sensing (QS), or chemical coordination of bacterial gene expression, exert strong effects on the cells of higher organisms, including mammals and other eukaryotes. For example, in the opportunistic pathogen Pseudomonas aeruginosa, the key QS molecule N-(3-oxo-dodecanoyl) homoserine lactone (C12) governs the production of virulence factors and biofilm formation, and it has also been found to induce pro- and anti-inflammatory immune responses in several mammalian cell types. This important finding may explain the establishment of persistent infections of P. aeruginosa in humans. However, at present these interactions are poorly defined at the molecular level, and the identification of a bona fide receptor for C12 remains a crucial step toward gaining a full understanding of P. aeruginosa – human relationships.
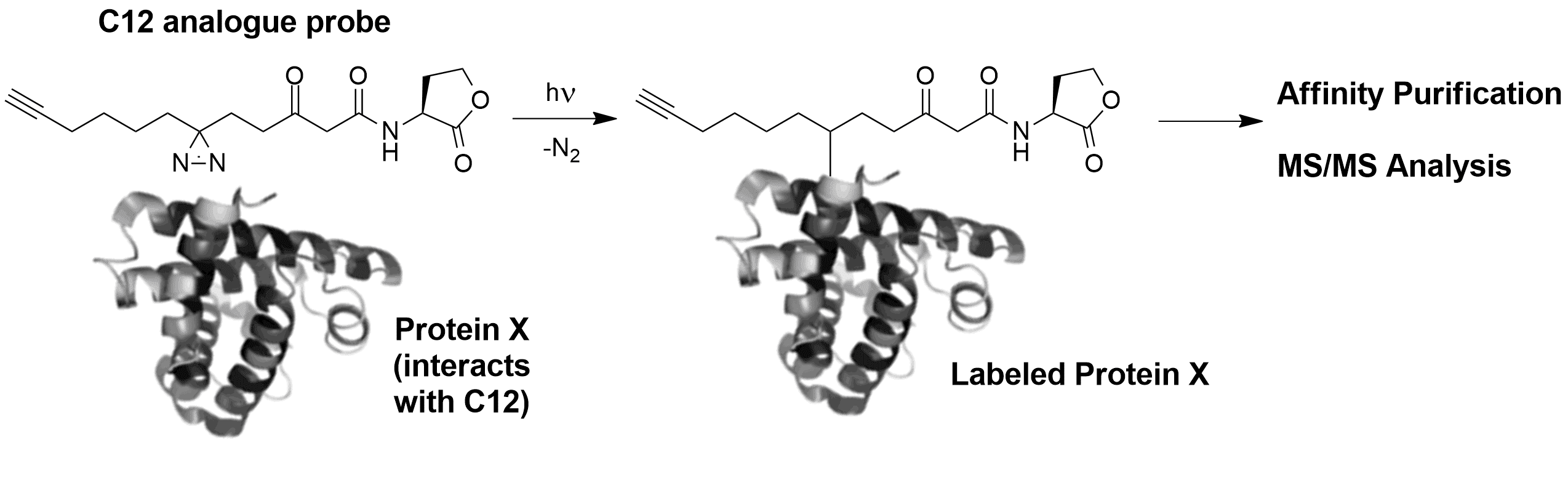
To elucidate such interkingdom interactions, our group has developed a tag-free photoaffinity labeling strategy involving the synthesis of C12 analogues with diazirine and alkyne moieties, in order to covalently bind target proteins in living cells upon irradiation. The labeled proteins are isolated using click-chemistry based affinity purification and analyzed by Orbitrap LC-MS/MS. To minimize false positives, cells are labeled using stable isotope labeling with amino acids in cell culture (SILAC). Using this methodology, we recently identified the major vault protein (MVP) as a putative receptor for C12. We examined the binding between our probe and purified vaults, and are currently exploring the effect in vivo of this protein on the innate immune response to C12.
Dubinsky, et al. Chem. Commun., 2013, 49, 5826-5828.
Dubinsky, et al.Chem. Commun., 2009, 47, 7378-80.
V. V. Kravchenko, et al. Science, 2008, 319, 5867, 1232-5.
Powered by Eventact EMS