
USING FRUSTRATED LEWIS PAIRS FOR THE SYNTHESIS OF NEW SILENES
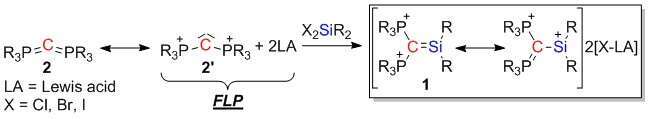
Frustrated Lewis pairs (FLPs) were shown to activate different small molecules, among these small molecules the activation of the Si-H bond in silanes drew a lot of attention due to their synthetic potential. We would like to use FLP`s approach to activate Si-X bonds (X = halogen) in dihalo-silanes in an attempt to prepare new functionalized silenes (1), molecules having Si=C double bond. To prepare the desired silene (1) we chose carbodiphosphorane (R3P=C=PR3 (2)) as Lewis base, due to two electron pairs residing on the carbon atom in the resonance form 2`. Preliminary DFT calculations using B3LYP/6-311G** level of theory of the parent system [(H3P)2C=SiH2]2+ provided promising results, wherein the dominant resonance structure indeed has a Si=C double bond. The chemistry of the desired silene is expected to be intriguing due to phosphonium functionalities attached to the carbon atom in 1. Herein theoretical predictions as well as progress in the synthesis of 1 are presented.
Powered by Eventact EMS