
Remote and modulably selective cleavage of ω-alkenyl spiropentane derivatives
Remote functionalization has becoming an emerging and increasingly popular concept in synthesis. Indeed, a series of pioneering publications have highlighted the transition-metal assisted remote functionalization of alkenes through tandem reactions involving olefin isomerization or chain-walking process.[i]
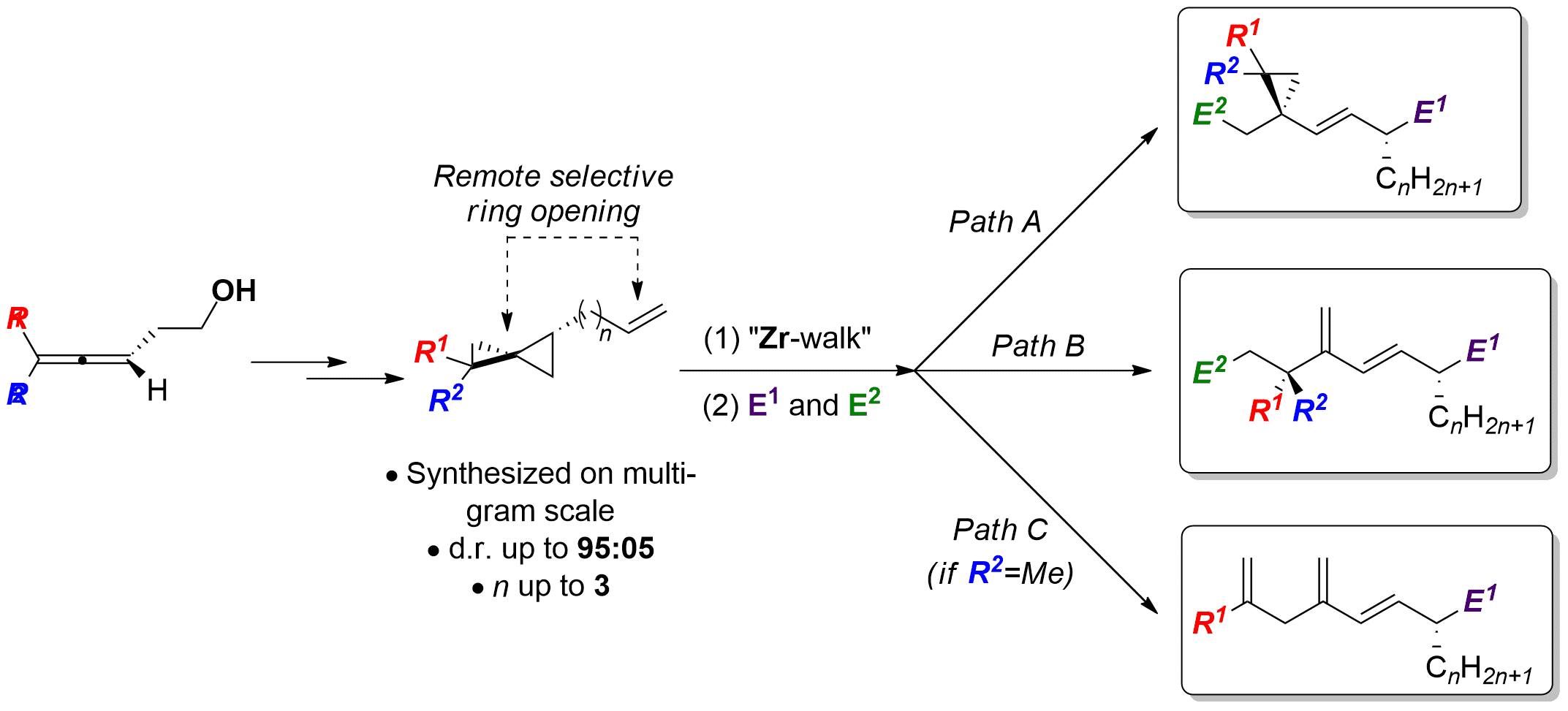
In our research group, we have achieved so far distant activations of alkenes using a low-valent zirconocene-butene complex generated in situ, namely the Negishi reagent. More specifically, we have reported the remote cleavage of ω-alkenyl cyclopropanes[ii] in one-pot procedures involving successively zirconium-walk[iii] (migration of the metal on a hydrocarbon chain), selective ring-opening of a three-membered ring, followed by a double functionalization reaction.
In the present context, we hereby report the modulable and selective cleavage of ω-alkenyl spiropentane derivatives, which are chemically accessible starting from allenes. Using organozirconium chemistry, we have set divergent methodologies for the synthesis of vinylcyclopropane, diene and triene derivatives in good yields with excellent stereoselectivities.
[i]. a) Grotjahn, D. B., Larsen, C. R., Gustafson, J. L., Nair R. & Sharma, J. Am. Chem. Soc. 129, 9592-9593 (2007); b) Ohlmann, D. M., Gooßen, L. J. & Dierker, M. Chem. Eur. J. 17, 9508-9519 (2011); c) Kochi, T., Hamasaki, T., Aoyama, Y., Kawasaki, J. & Kakiuchi, F. J. Am. Chem. Soc. 134, 16544-16547 (2012); d) Mei, T. –S, Patel, H. H. & Sigman, M. S Nature 508, 340-344 (2014).
[ii]. Masarwa, A., Didier, D., Zabrodsky, T., Schinkel, M., Ackermann, L. & Marek, I. Nature 505, 199-203 (2014).
[iii]. Marek, I., Chinkov N. & Levin, A. Synlett 501-514 (2006).
Powered by Eventact EMS