
STEREOSELECTIVE FORMATION OF TRISUBSTITUTED KETONE ENOLATES: A NEW ROUTE TO QUATERNARY STEREOCENTERS
The carbonyl group is one of the most versatile and broadly utilized functional groups in organic synthesis particularly through the preparation of enolate for carbon-carbon and carbon-heteroatom bonds formation. In view of the tremendous utility of such enolates in synthesis, application of this chemistry to the stereoselective synthesis of quaternary carbon stereocentres1 from stereochemically defined acyclic trisubstituted enolates of ketones would be highly valuable. However, the main problem that limits the formation of such intermediates is their regio- and stereoselective synthesis.2
Herein, we are pleased to report a new approach to the preparation of a single regioisomer and stereoisomer of stereodefined trisubstituted ketone enolate such as 1 (R1 ≠ R2 ≠ R3 = alkyl), in a single-pot operation from simple starting materials.3
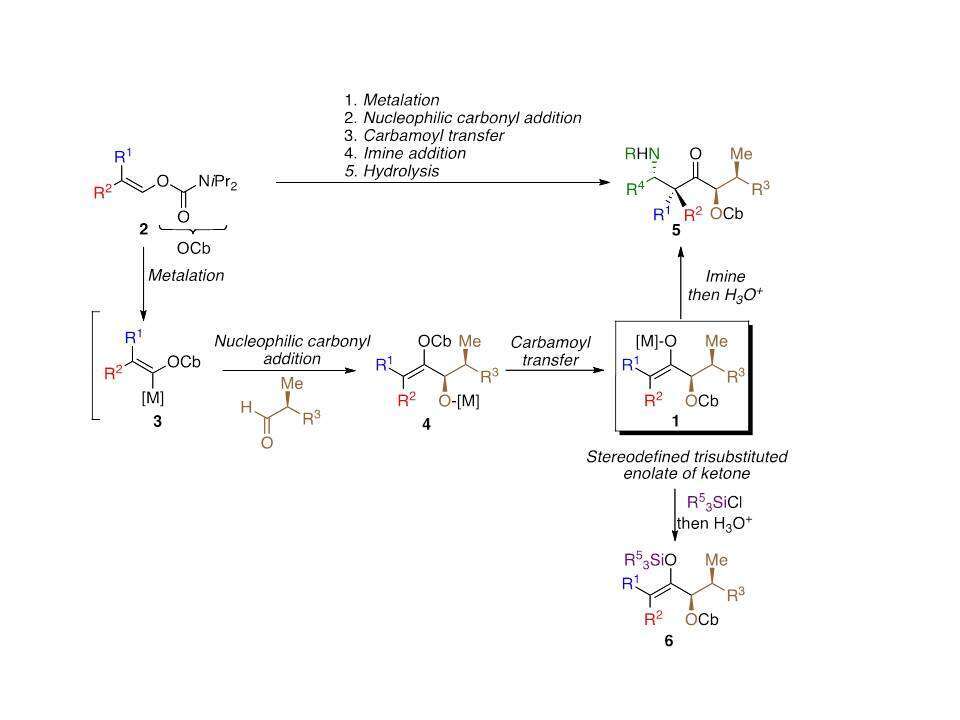
This completely new approach, that do not start by classical enolization processes, should present the advantage to create several carbon-carbon bonds in a single-pot operation with the formation of more than one stereogenic centers, including the quaternary carbon stereocenter as summarized in Figure 1.
- Minko Y., Pasco M., Lercher L., Botoshansky M., Mark I..Nature 490,522-526 (2012).
- Marek, I. et al. Am. Chem. Soc. 136, 2682-2694 (2014).
- Haimov E., Nairoukh Z., Shterenberg A., Berkovitz T., Jamison T., Marek I. Manuscript submitted.
Powered by Eventact EMS