
NEW (IMIDAZOLYLMETHYLAMINO)METHYL PHOSPHONATE ANALOGUES AS Zn(II) CHELATORS: SYNTHESIS AND CHARACTERIZATION
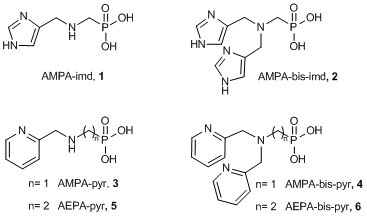
With a view to developing new, water soluble, high-affinity Zn(II)-chelators, we designed (imidazolylmethylamino)methyl phosphonate derivatives and explored their stoichiometry, acid-base equilibrium and stability constants of their complexes with Zn(II)-ions. AMPA-imd, 1, and AMPA-bis-imd, 2, were both obtained in a one pot reaction starting from aminomethylphosphonic acid and 4-formyimidazole to give the Schiff’s base intermediate, which was further reduced by 2-picoline borane to obtain both products 1 and 2 in 23% and 14%, respectively. Compounds 3-4 and 5-6 were synthesized from aminomethylphosphonic acid and aminoethylphosphonic acid, respectively, treated with 2-(bromomethyl)pyridine and NaOH in water, at 70 oC. Products 3, 4, 5, and 6 were obtained in 38%, 23%, 25%, and 15%, respectively. Acidity constants were established for each derivative by potentiometric pH-titrations. For instance, pKa values of AMPA-imd, 1, and AMPA-bis-imd, 2, were 3.9, 5.5, and 9.9, and 1.7, 6.0, and 9.6, respectively. The stoichiometry of complexes of 1 and 2 with Zn(II) was found to be 2:1, ML2, as established by Zn(II) titration monitored by both UV (Yoe and Jone’s method) and 1H- and 31P-NMR measurements. In addition, according to NMR measurements, the Zn(II)-coordination sites were mainly the amine and the imidazole ring(s) while the phosphonate moiety is probably not involved in coordination. Stability constants of the Zn(II) complexes of 1 and 2 were determined as logK(ML2)=11.3 and 13.7, respectively. In conclusion, the presented chelators tend to form ML2 complexes with Zn(II), where Zn(II) is coordinated by the amine and imidazole moieties. Compounds 1 and 2 have a high affinity towards Zn(II)-ions, where compound 2 has 251-fold higher affinity due to the presence of an additional imidazole ring.
Powered by Eventact EMS