Immunochemistry is a powerful tool, based on the concept that specific antigens can be detected by complementary antibodies. In the last few decades the use of colloidal gold in transmission electron microscopy has grown at an enormous rate, and has become virtually the only method worth considering for ultrastructural studies of cellular antigens. Immunogold labeling takes advantage of the high electron density of gold conjugated to antibodies, which in turn adsorb to a specific antigen. Although the probing molecules are often antibodies, other proteins of a specific affinity can be tagged.
Phosphatidylserine (PS) is a negatively charged phospholipid, found mostly in the inner leaflet of the cell membrane, facing the cytoplasm. Certain cell processes, such as apoptosis and microparticle shedding, involve PS migration to the outer leaflet. This phenomenon can be studied using the cellular protein, annexin V, which has high affinity to PS. Annexin V binding is Ca2+-dependent, which should be taken into account during the procedure.
Liposomes are spheroidal vesicles, composed of one or more lipid bilayers. They allow the easy and fairly realistic mimicking of bio-membranes; they can be built-up by one or just a few well-defined components, and hence allow better characterization of the physical principles underlying the self-organization processes observed in membranes. Immunogold labeling of PS in mixed lipid bilayer will significantly contribute to the study of the nano-scale domain formation mechanism. Until now these domains have been studied by direct imaging only on the micrometer scale, not on the nanoscopic scale. Understanding this phenomenon is important, because they are involved in a variety of cellular functions and biological events.
Thus far, most immunogold labeling has been performed in ways, which eventually lead to room temperature imaging by TEM or SEM. Our work presents immunogold labeling in cryo-TEM. Cryo-TEM preserves the liposomes as close as possible to their native state, thus providing a more reliable view of the nanostructure and its morphology. We attempted to label PS liposomes prepared by sonication and extrusion. Labeling was performed in solution, using biotinilated annexin V and gold-conjugated streptavidin, in a two-step process. We optimized the working conditions leading to extensive labeling of PS. The gold nanoparticles were not randomly spread in the solution, suggesting that the immunogold labeling in cryo-TEM was indeed successful. Apart from the excellent labeling we observed aggregation, which we attributed to the increased concentration of Ca2+ ions in the vicinity of the highly charged liposomes. The successful labeling of liposomes allows the application of the methodology to synthetic mixed-lipid and natural systems, such as extracellular vesicles.
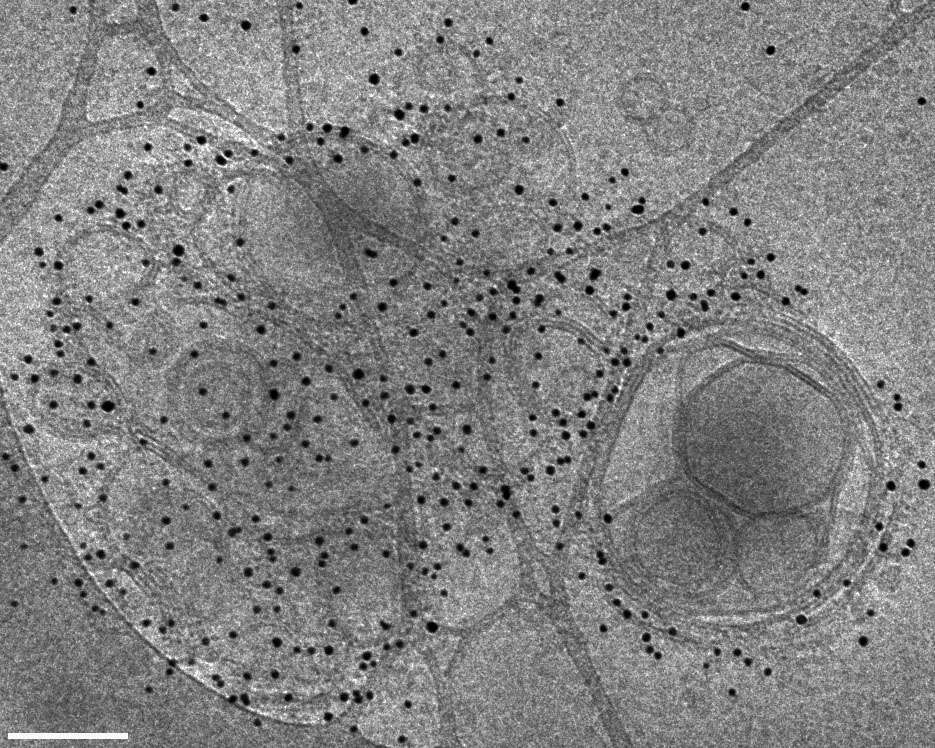
Figure 1: Cryo-TEM image of DOPS vesicles dispersion in PBS buffer (pH 7.4). These vesicles were prepared by sonication at room temperature, and were labeled in solution by biotinilated annexin V and 5 nm gold conjugated Streptavidin. Bar = 100 nm.

